Mechanical thrombectomy for acute ischemic stroke (AIS) has evolved dramatically over the past decade, during which time results from 6 randomized controlled trials of early thrombectomy1-6 and 2 of later thrombectomy7,8 have established mechanical thrombectomy as the standard of care for patients with AIS harboring a large vessel occlusion (LVO). Meta-analyses9,10 provide invaluable insights into the effect of thrombectomy on patients with LVO strokes. The updated 2018 American Heart Association/American Stroke Association (AHA/ASA) and European Stroke Organization guidelines have quadrupled the therapeutic time window and support thrombectomy for select LVO strokes up to 16 (Class I) to 24 (Class IIa) hours from time last known well (TLKW).11
Because approximately 30% of patients with AIS have LVO, mechanical thrombectomy has immense potential to prevent severe disability and mortality.12 Every aspect of stroke care from first medical contact to long-term post-stroke recovery is a window of opportunity to increase thrombectomy utilization and improve functional outcomes. It is critical to implement efficient care systems and appropriate triage of patients with AIS using a simplified and permissive patient selection paradigm. This must complement rapid adoption of new devices and techniques for thrombectomy designed to achieve high-quality recanalization with minimum complications. Further understanding of heterogeneity in stroke pathophysiology along with emerging neuroprotective therapies will affect triage, treatment, and ultimately outcomes for those with LVO strokes.
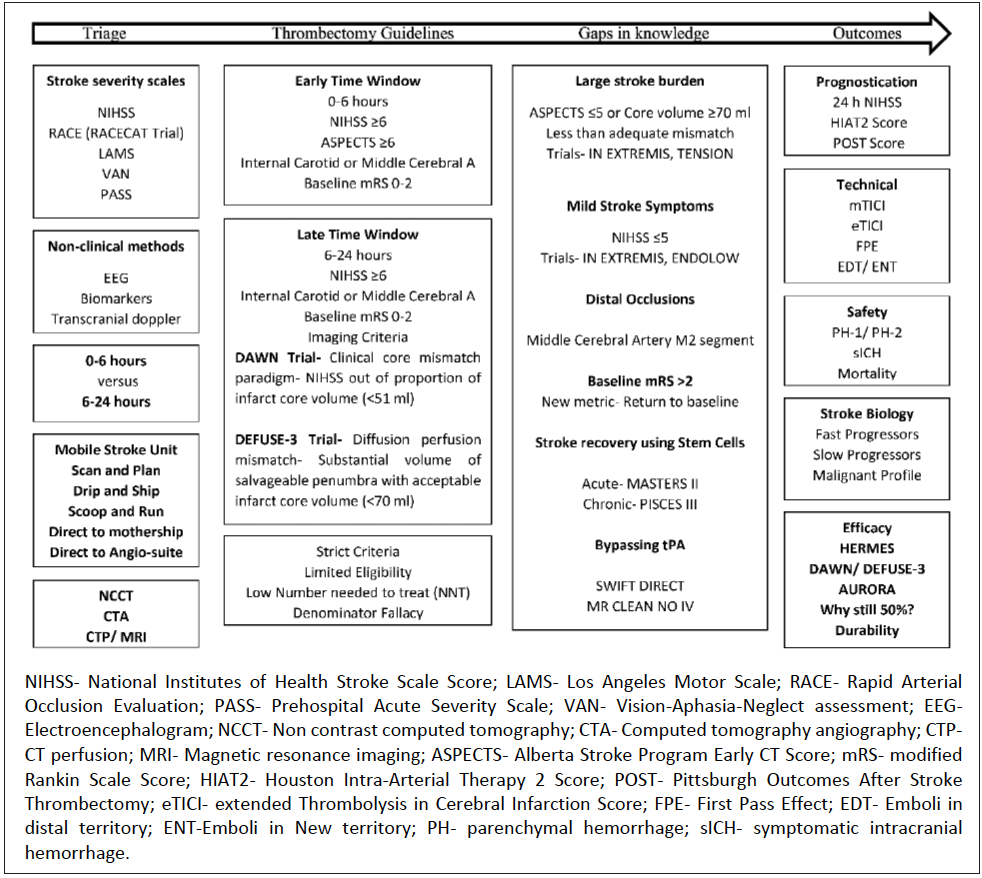
This article provides a review of the updated AHA/ ASA guidelines for management of patients with LVO strokes with an overview of the recently published late-window clinical trials, DAWNa and DEFUSE-3b, and how this information affects patient selection and outcomes. Opportunities to extend the scope of thrombectomy to subgroups yet not studied in clinical trials exist and are the focus of ongoing and upcoming trials. New metrics for technical success of thrombectomy and adjudication of good outcomes are also discussed. Last, the importance of stroke heterogeneity and the existence of distinct stroke phenotypes (fast versus slow progressors) is highlighted (Figure 1).
Prehospital Triage
Prehospital triage of all patients with AIS to a primary stroke center vs a thrombectomy-capable stroke center depends on various factors: likelihood of LVO, time window of presentation, efficacy of tissue plasminogen activator (tPA) for proximal anterior circulation occlusions, and transport time (See Triage For A Higher Level of Care in this issue).
Patient Selection
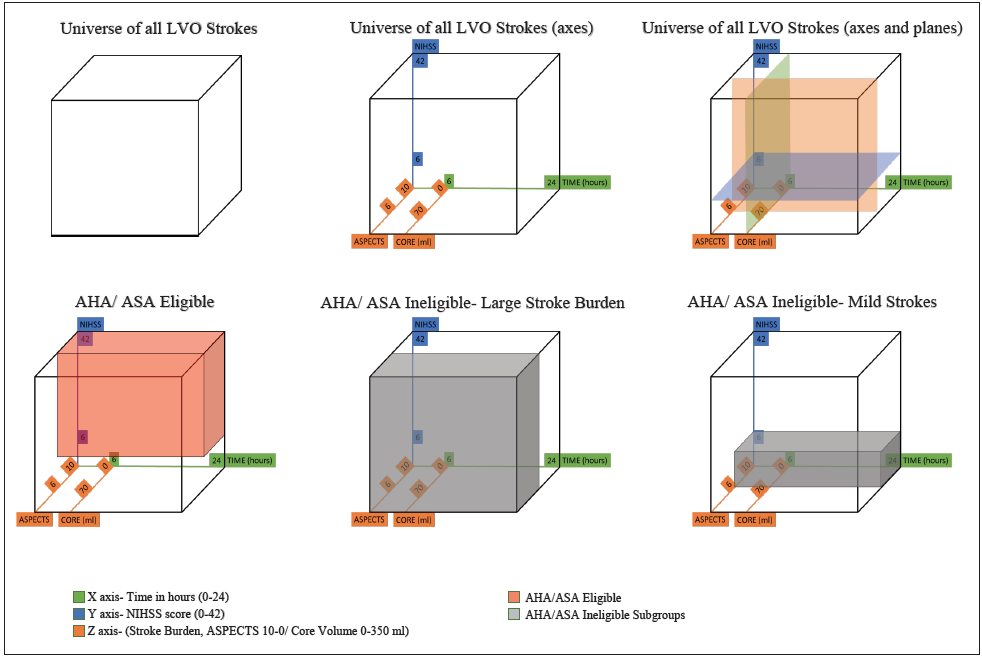
Patient selection for mechanical thrombectomy is guided in part by criteria used in recent trials and subsequent recommendations provided by stroke association guidelines.11 Center-specific patient-selection criteria may be broader and depend on conversations between the vascular neurologist, neuro-interventionalist, and the patient and family. Per AHA/ASA guidelines, a patient with AIS who presents within 6 hours may be selected for mechanical thrombectomy with the following demographic criteria: age 18 years or more, NIHSS score 6 or above, and a baseline modified Rankin Scale (mRS) score between 0 to 2. Noncontrast computed tomography (NCCT) and CT angiography (CTA) should be performed to rule out a large territory stroke (< 6) and confirm an anterior circulation LVO (internal carotid or middle cerebral artery [MCA]). Patient selection in the later time window—more than 6 hours—is more complex. Advanced imaging with CT perfusion studies (CTP) or MRI can help delineate an established infarct core and potential mismatch (clinical-core mismatch defined by the DAWN trial, target mismatch defined by the DEFUSE-3 trial).11 Selection in the late-time window may also be simplified—the feasibility of which is supported by retrospective studies.13,14
Eligibility of patients with AIS for thrombectomy in the early and late time window per AHA/ASA criteria is only 11% and 9%, respectively.15,16 The number needed to treat (NNT) in the HERMESc, DAWN, and DEFUSE-3 trials were 5, 2.8, and 3.6, respectively. Limited eligibility (~10%) and large treatment effect (low NNT) suggests that AHA/ASA recommendations are restrictive and some patients who may benefit from thrombectomy may not meet top tier criteria. The decision to treat only the most likely to benefit patients and demonstrate efficacy was an important first step; however, this severely limits the overall benefit of this therapy17 and is at odds with patient preferences.18 It may now be time to consider how to treat the most patients, maximize benefit across all patients with LVO, and reduce overall disability due to stroke. The hesitation of expanding the patient selection criteria is likely due to the perception of increased harm (eg, hemorrhage) in a minority of patients. The authors of this article posit that the outcome would not change in this minority, given the unfavorable natural history of LVO. Limiting therapy would likely expand the number of patients with disability and unfavorable long-term outcome. Broadening the scope of thrombectomy requires extending therapy to types of patients who were not included in clinical trials, including those with a large stroke burden on presentation (ASPECTS score ≤ 5), mild symptoms (NIHSS score ≤ 5), distal occlusions (MCA M2 section and beyond), and strokes with baseline mRS score greater than 2 (Figure 2).
Procedural Considerations
With increasing thrombectomy utilization and operator experience as well as improving safety profile and thrombectomy devices and technique efficacy, the procedural risk of thrombectomy will continue to diminish. Stent retrievers are recommended for thrombectomy with Class Ia evidence. A meta-analysis including 4 thrombectomy trials where stent retrievers were used confirmed the safety of stent retrievers with comparable rates of symptomatic intracranial hemorrhage as well as efficacy with successful recanalization rates (77%).19 Balloon-guided catheters (BGCs) may have higher successful recanalization rates,20 although this has not been demonstrated in a randomized controlled trial setting.
New metrics to adjudicate successful recanalization are being developed. The most widespread scoring system for the degree of recanalization, the modified Thrombolysis in Cerebral Infarction Scale (mTICI) has limited interrater reliability and a large magnitude of difference between the degree of recanalization defined by score 2b and 2c/3. A more granular score has been developed and tested, the extended TICI (eTICI) score,21 which can delineate meaningful clinical differences in outcomes based on the degree of recanalization. Another metric for recanalization and an independent predictor of good outcome is the first pass effect (FPE), defined as achievement of complete recanalization (TICI 3) after the first thrombectomy attempt.22 The FPE is associated with the use of BGC and occlusion location does not involve the internal carotid artery (ICA).
Other interprocedural safety metrics are embolization in new territory (ENT), defined as emboli observed on post-thrombectomy angiography within previously unaffected territories, and embolization in distal territory (EDT), defined as emboli seen within the territory of the vessel where the thrombus was originally located both of which can increase procedure time and technical challenges, leading to higher ischemic insult and decreasing likelihood of a good outcome.23,24 Conventional guide-wire—based stent retriever thrombectomy without concurrent aspiration may increase the rates of ENT/EDT. New devices such as EmboTrap II (Cerenovus; with an inner channel inside the stent retriever) may lead to lower rates of ENT/EDT. Using aspiration as first-line thrombectomy technique has been demonstrated to be as good as using a stent retriever.25-27
Prognosis
Functional independence rates (mRS 0-2) 90 days after AIS due to LVO have increased significantly since the advent of thrombectomy. Thrombectomy outcomes are dependent on age and NIHSS score and ischemic core volume are inversely related to the likelihood of a good outcome.9,28 Prognostication scores like the Pittsburgh Outcomes After Stroke Thrombectomy (POST) Score29 and Houston Intra-Arterial Therapy 2 (HIAT2) score30 have been developed to predict outcome after anterior circulation stroke thrombectomy. Outcomes after thrombectomy are highly dependent on time, particularly in the early-time window. The effect of time exists but is less profound in the late-time window because of patient selection emphasizing those with small ischemic cores and presence of salvageable brain tissue.31,32 Recent data indicate the rate of neuron loss in patients with LVO is highly variable, ranging from less than 35,000 to more than 27 million neurons per minute.33
With respect to outcomes, it is important to consider why only 50% of patients with LVO who have thrombectomy become functionally independent, whether data exists to support thrombectomy in patients with LVO stroke types not studied in clinical trials, and what entails a “good outcome.” The next frontier for mechanical thrombectomy is to further optimize outcomes. Research is ongoing regarding efforts to shorten times to groin puncture, achieve high quality recanalization using new devices and techniques, understand stroke biology for effective triage and treatment (slow versus fast progressors), explore neuroprotective strategiesd, minimize edemae, and reduce rates of parenchymal and symptomatic intracranial hemorrhage.34 Introduction of stem cells in the acutef and chronicg recovery periods after ischemic stroke are exciting opportunities in the management of patients with AIS who have mechanical thrombectomy.
Conclusions
Current guidelines regarding thrombectomy for patients with AIS are restrictive. Important types of patients with LVO stroke do not meet AHA/ASA criteria. Data from meta-analysis of 7 thrombectomy trials indicate that mechanical thrombectomy in stroke involving more than one-third of the MCA territory or ASPECTS score of 6 or under provides benefit despite increase in the rate of symptomatic intracranial hemorrhage.35 Further analysis of patients who underwent CT perfusion indicates that ischemic core is an independent predictor of functional independence and clinically meaningful treatment effect of thrombectomy exists with increasing ischemic core size.36 Retrospective data regarding thrombectomy in people with mild strokes with LVO is inconclusive.37,38 Equipoise therefore exists and randomized controlled trials are underway including for people with large strokesh, mild deficits, and mild strokesi. Results of these trials may help further expand the indication of mechanical thrombectomy.
The individual’s definition of favorable outcome is important but difficult to elucidate in randomized clinical trials. Functional independence is appropriately used as an efficacy end-point in trials and clinical practice; however, a patient with a large stroke burden or baseline function of mRS 3 may accept mRS 3 or return to baseline as a good outcome. In contrast, in patients with low NIHSS score and distal occlusions, cognition39 or an mRS from 0 to 1 may be a more meaningful measurement of a good outcome.
The field of interventional neurology is expanding rapidly. The incidence and prevalence of LVO stroke and accompanying disability is increasing with a global demographic shift towards the elderly. Mechanical thrombectomy is a powerful tool to treat people with LVO strokes and alter its devastating natural history. Maximizing the potential of this treatment is essential.
a. Clinical mismatch in the triage of wake up and late presenting strokes undergoing neurointervention with Trevo (NCT02142283).
b. Endovascular therapy following imaging evaluation for ischemic stroke (NCT02586415).
c. Highly effective reperfusion evaluated in multiple endovascular stroke trials.
d. Safety and efficacy of NA-1 in subjects undergoing endovascular thrombectomy for stroke (NCT02930018).
e. Phase 3 study to evaluate the efficacy and safety of intravenous BIIB093 (glibenclamide) for severe cerebral edema following large hemispheric infarction (NCT02864953).
f. Multistem administration for stroke treatment and enhanced recovery study (NCT03545607).
g. Pilot investigation of stem cells in stroke (NCT02117635).
h. Efficacy and safety of thrombectomy in stroke with extended lesion and extended time window (NCT03094715).
i. In extremis study—minor stroke therapy evaluation—NIHSS 0-5 and large stroke therapy evaluation—ASPECT 0-5.
1. Berkhemer OA, Fransen PSS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11-20.
2. Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296-2306.
3. Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019-1030.
4. Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285-2295.
5. Campbell BCV, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009-1018.
6. Bracard S, Ducrocq X, Mas JL, et al. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. 2016;15:1138-1147.
7. Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11-21.
8. Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection byperfusion imaging. N Engl J Med. 2018;378:708-718.
9. Goyal M, Menon BK, Zwam WH van, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. The Lancet. 2016;387:1723-31.
10. Jovin TG, Lansberg MG, Hill MD, et al. Thrombectomy for anterior circulation stroke beyond 6 hours from time last known well: primary results of AURORA (Individual patient level pooled-analysis from five randomized trials. Eur Stroke J. 2018;3(IS):6.
11. Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46-e110.
12. van Seeters T, Biessels GJ, Kappelle LJ, et al. The prognostic value of CT angiography and CT perfusion in acute ischemic stroke. Cerebrovasc Dis Basel Switz. 2015;40:258-269.
13. Desai SM, Rocha M, Molyneaux BJ, et al. Thrombectomy 6-24 hours after stroke in trial ineligible patients. J Neurointerv Surg. 2018;10(11):1033-1037.
14. Vanacker P, Lambrou D, Eskandari A, Mosimann PJ, Maghraoui A, Michel P. Eligibility and predictors for acute revascularization procedures in a stroke center. Stroke. 2016;47:1844-1849.
15. Jadhav AP, Desai SM, Kenmuir CL, et al. Eligibility for endovascular trial enrollment in the 6- to 24-Hour time window. Stroke. 2018; 49(4):1015-1017.
16. Goyal M, Jadhav AP. Denominator fallacy revisited. J Neurointerv Surg. 2017;9 915-916.
17. Wang F, Campbell BCV, Churilov L, et al. Insights into variations in preferred selection criteria for acute stroke endovascular therapy. J Neurointerv Surg. 2018;10:542-549.
18. Campbell BCV, Hill MD, Rubiera M, et al. Safety and Efficacy of Solitaire Stent Thrombectomy: Individual Patient Data Meta-Analysis of Randomized Trials. Stroke. 2016;47:798-806.
19. Zaidat O, Liebeskind D, Jahan R, et al. O-005 Influence of Balloon, Conventional, or Distal Catheters on Angiographic and Technical Outcomes in STRATIS. J Neurointerv Surg. 2016;8:A3-A4.
20. Liebeskind DS, Bracard S, Guillemin F, et al. eTICI reperfusion: defining success in endovascular stroke therapy. J NeurointervSurg 2018; pii:neurintsurg-2018-014127.
21. Zaidat OO, Castonguay AC, Linfante I, et al. First pass effect: a new measure for stroke thrombectomy devices. Stroke. 2018;49:660-666.
22. Mazur MD, Kilburg C, Park MS, Taussky P. Patterns and clinical impact of angiographically visible distal emboli during thrombectomy with solitaire for acute ischemic stroke. Neurosurgery. 2016;78:242-250.
23. Mokin M, Setlur Nagesh SV, Ionita CN, Levy EI, Siddiqui AH. Comparison of modern stroke thrombectomy approaches using an in vitro cerebrovascular occlusion model. AJNR Am J Neuroradiol. 2015;36:547-551.
24. Zaidat OO, Bozorgchami H, Ribó M, et al. Primary results of the multicenter ARISE II study (Analysis of Revascularization in Ischemic Stroke With EmboTrap). Stroke. 2018;49:1107-1115.
25. Chueh J-Y, Puri AS, Wakhloo AK, Gounis MJ. Risk of distal embolization with stent retriever thrombectomy and ADAPT. J NeurointervSurg 2014; neurintsurg-2014-011491.
26. Turk AS, Siddiqui AH, Mocco J. A comparison of direct aspiration versus stent retriever as a first approach (‘COMPASS’): protocol. J NeurointervSurg. 2018;10:953-957.
27. Lapergue B, Blanc R, Gory B, et al. Effect of endovascular contact aspiration vs stent retriever on revascularization in patients with acute ischemic stroke and large vessel occlusion: the ASTER randomized clinical trial. JAMA. 2017;318:443-452.
28. Saver JL, Goyal M, Lugt A van der, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: A meta-analysis. JAMA. 2016;316:1279-1289.
29. Rangaraju Srikant, Liggins John T.P., Aghaebrahim Amin, et al. Pittsburgh outcomes after stroke thrombectomy score predicts outcomes after endovascular therapy for anterior circulation large vessel occlusions. Stroke. 2014;45:2298-2304.
30. Sarraj A, Albright K, Barreto AD, et al. Optimizing prediction scores for poor outcome after intra-arterial therapy in anterior circulation acute ischemic stroke. Stroke. 2013;44:3324-3330.
31. Rocha M, Jovin TG. Fast versus slow progressors of infarct growth in large vessel occlusion stroke: clinical and research implications. Stroke. 2017;48:2621-2627.
32. Rocha M, Desai SM, Jadhav AP, Jovin TG. Distribution and incidence of fast versus slow progressors of infarct growth in large vessel occlusion stroke. ISC 2018, 2018.
33. Desai SM, Rocha M, Jovin TG, Jadhav AP. High variability in rate of neuronal loss-time is brain, re-quantified. Stroke Press 2018;published online Dec.
36. Lyden P, Pryor KE, Coffey CS, et al. Final Results of the RHAPSODY trial: A multi-center,phase 2 trial using a continual reassessment method to determine the safety and tolerability of 3K3A-APC, a recombinant variant of human activated protein C, in combination with tissue plasminogen activator, mechanical thrombectomy or both in moderate to severe acute ischemic stroke. Ann Neurol. 2018;published online Nov 18. doi:10.1002/ana.25383.
35. Román LS, Menon BK, Blasco J, et al. Imaging features and safety and efficacy of endovascular stroke treatment: a meta-analysis of individual patient-level data. Lancet Neurol. 2018;17:895-904.
36. Campbell BCV, Majoie CBLM, Albers GW, et al. Penumbral imaging and functional outcome in patients with anterior circulation ischaemic stroke treated with endovascular thrombectomy versus medical therapy: a meta-analysis of individual patient-level data. Lancet Neurol. 2018; 2018 Nov 6. pii: S1474-4422(18)30314-4.
37. Nagel Simon, Bouslama Mehdi, Krause Lars U., et al. Mechanical thrombectomy in patients with milder strokes and large vessel occlusions. Stroke. 2018;49:2391-2397.
38.Sarraj Amrou, Hassan Ameer, Savitz Sean I., et al. Endovascular thrombectomy for mild strokes: how low should we go? Stroke. 2018;49:2398-2405.
39. López-Cancio E, Jovin TG, Cobo E, et al. Endovascular treatment improves cognition after stroke: a secondary analysis of REVASCAT trial. Neurology. 2017;88:245-251.
Disclosures
The authors report no financial or other relationships relevant to this content.