Answer endocrine treatment planning questions with assurance
Answer endocrine treatment planning questions with assurance
One Platform.
Multiple Answers.
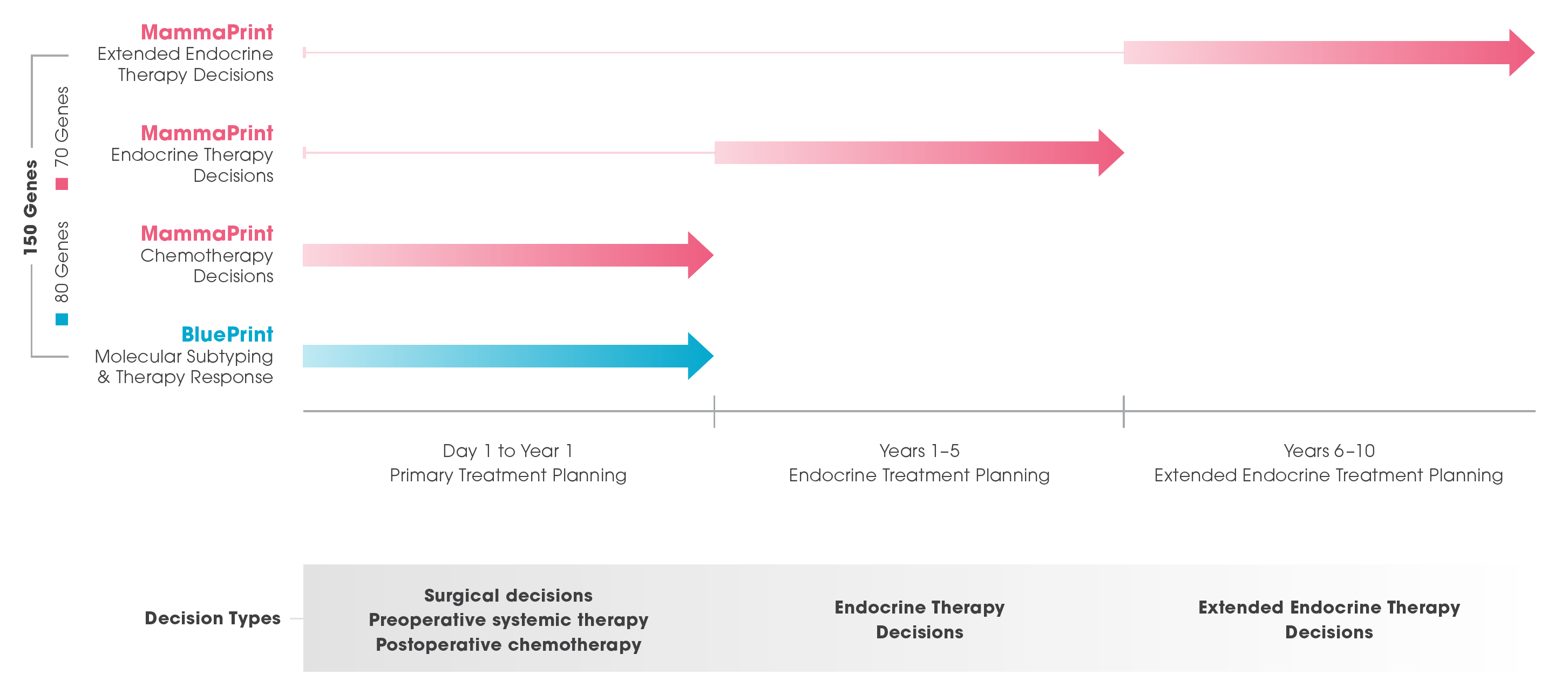
In a single genomic test – and as part of the most comprehensive platform available to support treatment planning for early-stage breast cancer – MammaPrint empowers clinicians with actionable information to answer important benefit questions regarding chemotherapy, standard of care endocrine therapy, and extended endocrine therapy.
MammaPrint with BluePrint means more.
Incorporating the unique utilities of both MammaPrint and BluePrint, Agendia’s breast cancer platform stands alone in its ability to identify the underlying biology of an individual breast cancer, then turn challenging planning questions into decisive, proven answers across a comprehensive range of breast cancer treatment decisions.
MammaPrint’s clear, actionable, proven risk stratification information equips clinicians to optimize treatment planning for up to 10 years with clinical rigor and personal confidence. BluePrint helps to identify a tumor’s molecular profile to define and reveal valuable information about its behavior and response to different systemic therapies.
Primary Treatment Planning Day 1 to Year 1
Order MammaPrint and BluePrint on the core biopsy sample for ER+, HER2- breast cancer patients.
- Make informed decisions about the timing of surgery
- Optimize pre-operative treatment based on the tumor’s molecular profile
- Know which patients will benefit from adjuvant chemotherapy, and which will not
Standard Endocrine Treatment Planning Years 1–5
- Identifies which patients will benefit from standard of care endocrine therapy compliance for the full five years
- Understand recurrence risk information, understand (and help patients understand) who can confidently discontinue endocrine therapy after 2 years, and who cannot
Extended Endocrine Treatment Planning Years 6–10
Order MammaPrint at any time in the first five years following diagnosis to determine the benefit of extended endocrine therapy.
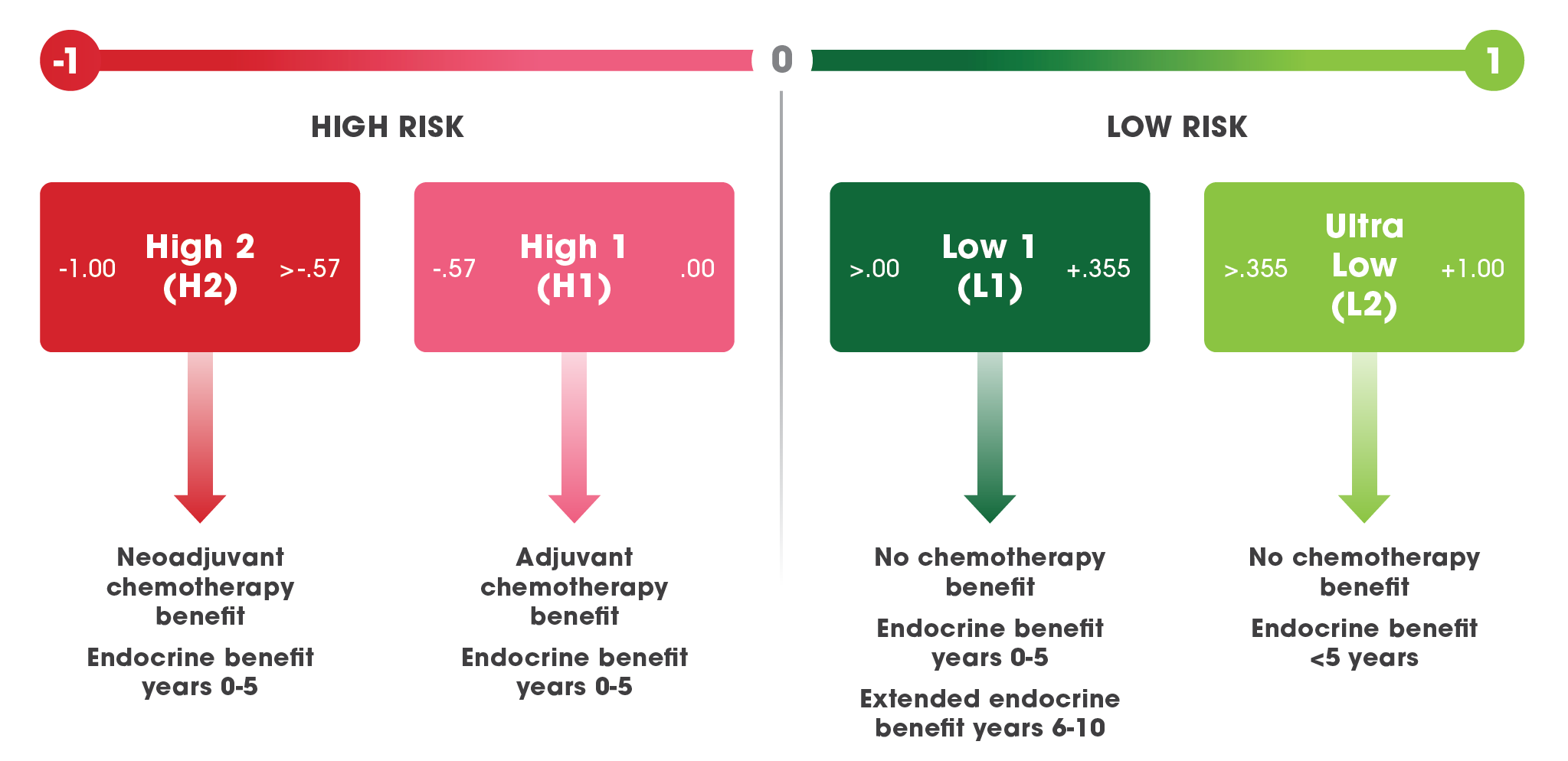
The molecular profiles for MammaPrint Ultra Low*, Low, and High Risk patient subgroups identify which patients will benefit from extended endocrine therapy and which will not.
MammaPrint is proven.
MammaPrint provides clinicians with the confidence of level 1 studies to predict personalized breast cancer outcomes for up to 20 years with and without chemotherapy, and with and without endocrine therapy.
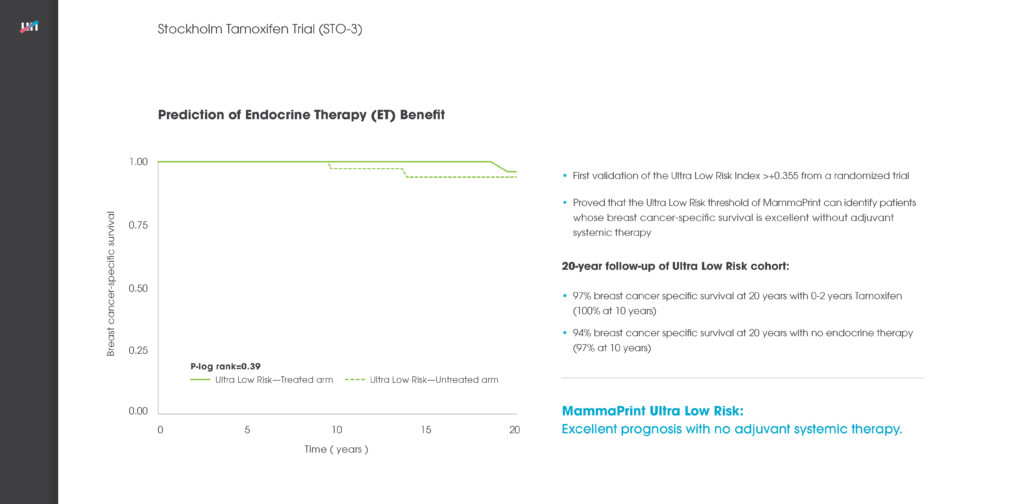
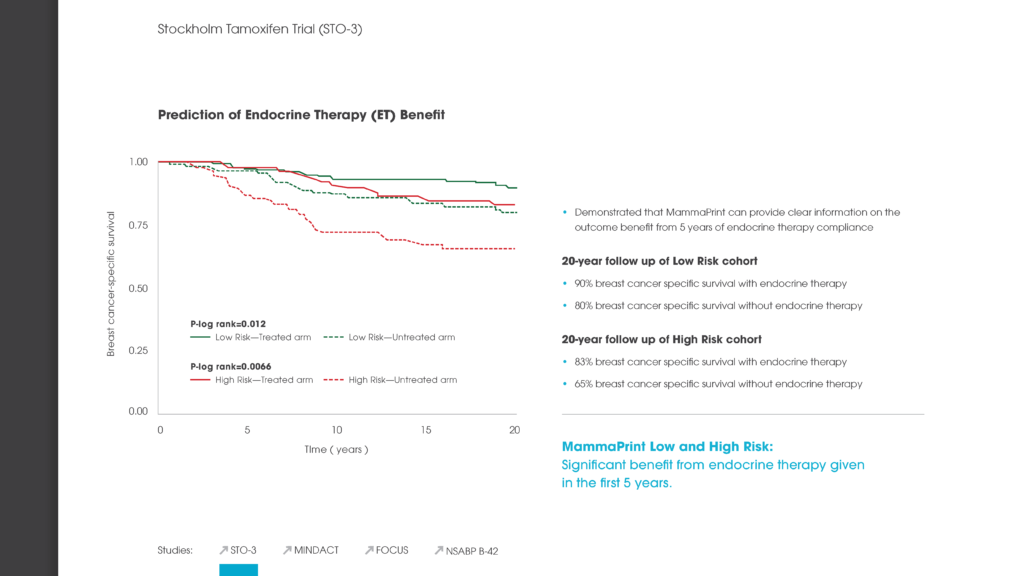
STO-3
Established MammaPrint as the only genomic test to show the benefit of endocrine therapy in Low and High Risk patients, and provided an initial validation of the Ultra Low Risk threshold.
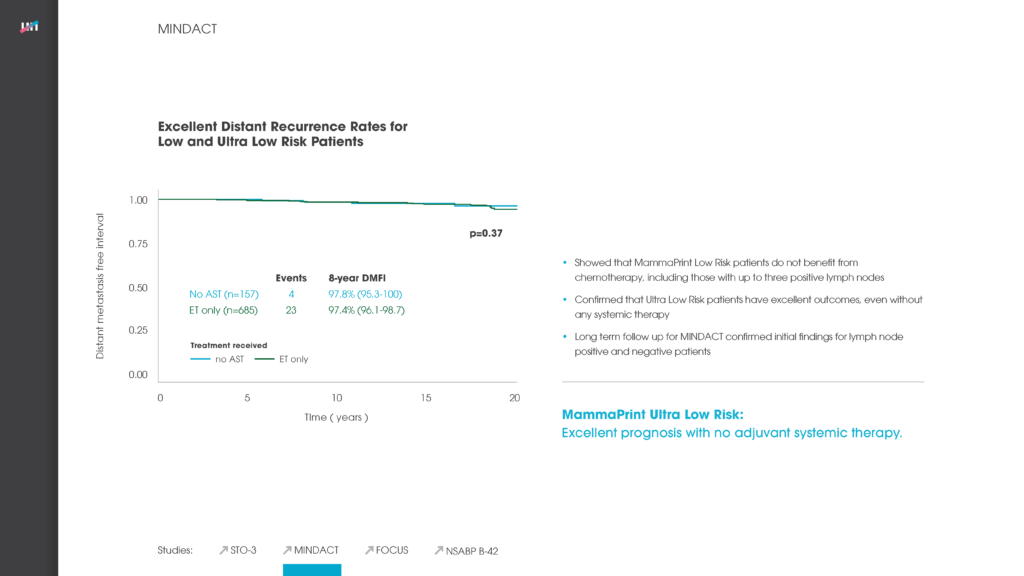
MINDACT
Showed that MammaPrint Low Risk patients do not benefit from chemotherapy, including those with up to three positive lymph nodes. Additionally, it confirmed that Ultra Low Risk patients have excellent outcomes, even without any systemic therapy..
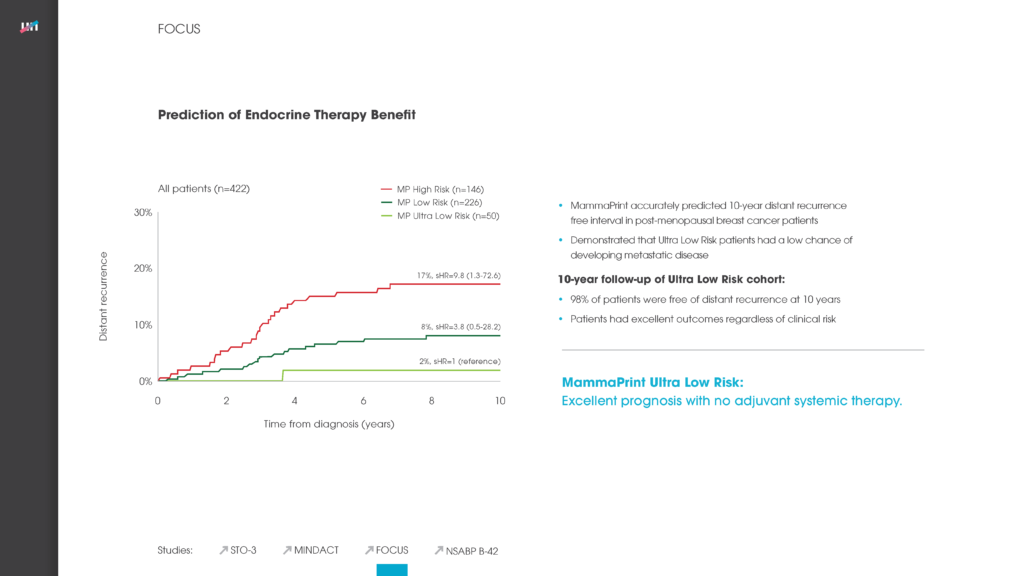
FOCUS
Confirmed that a MammaPrint Ultra Low Risk result identifies a subset of patients with extremely indolent cancer who may be candidates for endocrine therapy de-escalation.
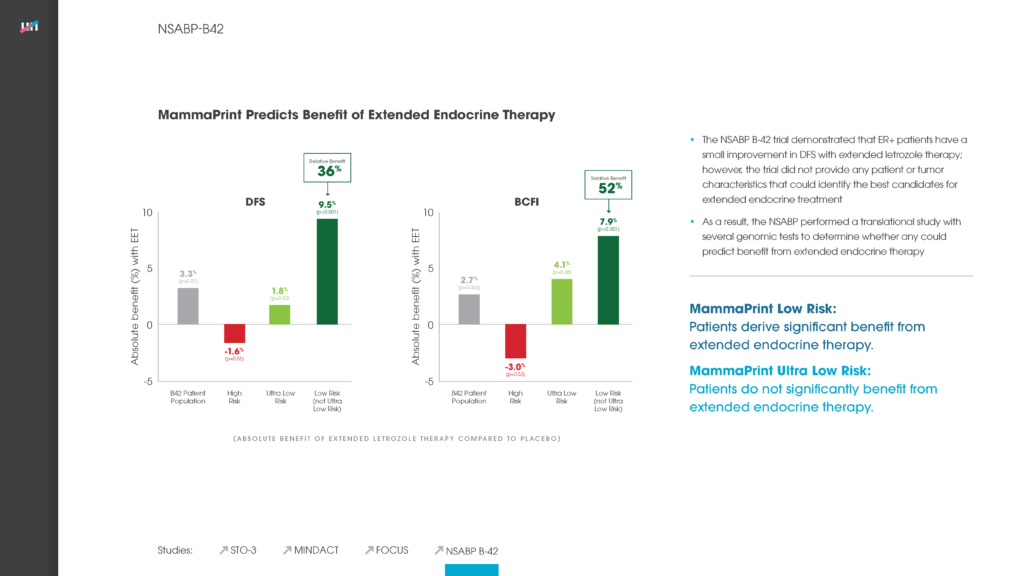
NSABP B-42
Found that Low (not Ultra Low) Risk patients are the only ones who derive significant benefit from extended endocrine therapy—thus advancing the value of MammaPrint for personalizing endocrine therapy.
MammaPrint is personalized.
Using a detailed molecular profile to define each patient’s unique cancer, MammaPrint’s 70-gene genomic testing equips clinicians to understand the personal patient benefit of chemotherapy and endocrine therapy with a single test. This provides ongoing, consistent, and confident treatment planning at time of diagnosis to avoid unnecessary over-treatment, while also ensuring full benefit for patients who need it.
MammaPrint is the only risk-of-recurrence test that segments ER+ patients into level 1 data-defined, clinically actionable sub-groups, each requiring different treatment management decisions through chemotherapy, endocrine therapy, and extended endocrine therapy.
MammaPrint Defines the Biology of ER+ Breast Cancer to Inform Management of Therapy
Want more information?
For the US, Puerto Rico, Canada and South America
Agendia Inc. USA
22 Morgan
Irvine, CA 92618
(888) 321-2732
customercare@agendia.com
For outside the Americas
Agendia NV
Radarweg 60
1043 NT Amsterdam
The Netherlands
customerservice@agendia.com
+31 (0)20 462 1510
*MammaPrint Ultra Low Risk is a subset within the Low Risk category.
Agendia’s laboratory is certified under the Clinical Laboratory Improvement Amendments (CLIA) as qualified to perform high-complexity clinical laboratory testing. MammaPrint and BluePrint have been CE-marked and are laboratory tests developed, validated and performed by Agendia.