In it for the Long Haul: More Questions than Answers on the Lasting Health Effects of COVID-19
General description and state of knowledge
Early in the pandemic, studies from China and Italy described a high prevalence of lasting symptoms among patients who had been hospitalized with COVID-19. Since then, data from a range of sources have advanced our understanding of the health problems experienced by some COVID-19 survivors. People who had what was classified as mild COVID-19 and were not hospitalized have spoken out about experiencing ongoing health effects. They have formed online groups, such as the Body Politic COVID-19 Support Group, and launched patient-led research initiatives. Such efforts increased scientific, medical and public recognition of the phenomenon of persistent health problems among COVID-19 survivors and coined the now widely used term “long COVID.”
A wide range of health problems and conditions have been reported as sequelae of COVID-19. The precise health effects people experience and names for those effects are still being defined; in addition to “long COVID,” other names include “post-COVID-19 syndrome,” “long-term COVID” and “chronic COVID syndrome,” and people who experience ongoing health problems after COVID-19 have been referred to as “long haulers.” The National Institutes of Health has launched an initiative to fund research on health effects that persist after COVID-19 and suggested that while these effects are still being defined, they may collectively be referred to as Post-Acute Sequelae of SARS-CoV-2 infection (PASC).
What causes Post-Acute Sequelae of SARS-CoV-2 infection (PASC)?
Before describing what PASC encompasses, it is useful to review what happens during acute infection with SARS-CoV-2, the virus that causes COVID-19. At the beginning of infection, the virus actively replicates in the body, and in the latter stages of infection the immune system clears the virus from the body. As we wrote in a previous In-Depth Science Review, symptoms can be caused both by direct effects of the virus and by an overactive immune response; treatments that dampen the immune response reduce the risk of death from severe COVID-19. The virus typically infects the respiratory tract and causes respiratory symptoms. The term “severe COVID-19” is generally applied to cases of acute infection when lung function has been impaired. However, many organs can be affected by the virus and the range of symptoms people with COVID-19 experience varies widely. Many of those who are infected may be asymptomatic, and they, too, can develop PASC. The relationship between the nature of acute infection and PASC is not yet well-understood.
Describing Post-Acute Sequelae of SARS-CoV-2 infection (PASC)
Timing
Most people recover from acute COVID-19 within weeks, but as with many diseases, recovery time may be longer in more severe cases. There is not yet consensus on where to draw the line between when COVID-19 ends and PASC begins. Various time courses have been proposed, including in guidelines on managing the long-term effects of COVID-19 from the National Institute for Health Care and Excellence in the United Kingdom. For the purposes of advising evaluation and management, those guidelines define “acute COVID-19” as lasting up to four weeks after diagnosis; “ongoing COVID-19” as lasting from four to 12 weeks after diagnosis; and “post-COVID-19 syndrome” as lasting for more than 12 weeks. There is increased recognition that the progression of PASC may be non-linear; in some cases, new symptoms arise after acute illness has resolved or as other symptoms dissipate. The variable timelines and short duration of many studies has limited our understanding of PASC; it has also only been about one year since the first reports of prolonged recovery from COVID-19 emerged. Indeed, a study that followed COVID-19 survivors for up to nine months provides one of the most long-term views of PASC.
Symptoms
Long-term symptoms among COVID-19 survivors may be similar or different to what was experienced during acute illness. Symptoms affecting nearly every part of the body have been reported. A preprint systematic review and meta-analysis of 15 studies on PASC that included more than 47,910 individuals identified 55 long-term effects potentially associated with COVID-19; these included a wide range of symptoms. The five most commonly reported symptoms were fatigue (58%), headache (44%), difficulty concentrating (sometimes described as “brain fog”) (27%), hair loss (25%), and shortness of breath (24%). The long-term effects reported in this analysis are represented in the figure below; some medical terms such as “ageusia” (loss of taste), “dyspnea” (shortness of breath) and “polypnea” (rapid breathing) are included. Reported symptoms are not just physical. In a study of more than 230,000 COVID-19 survivors, 13% received a new neurologic or psychiatric diagnosis within the first six months after diagnosis.
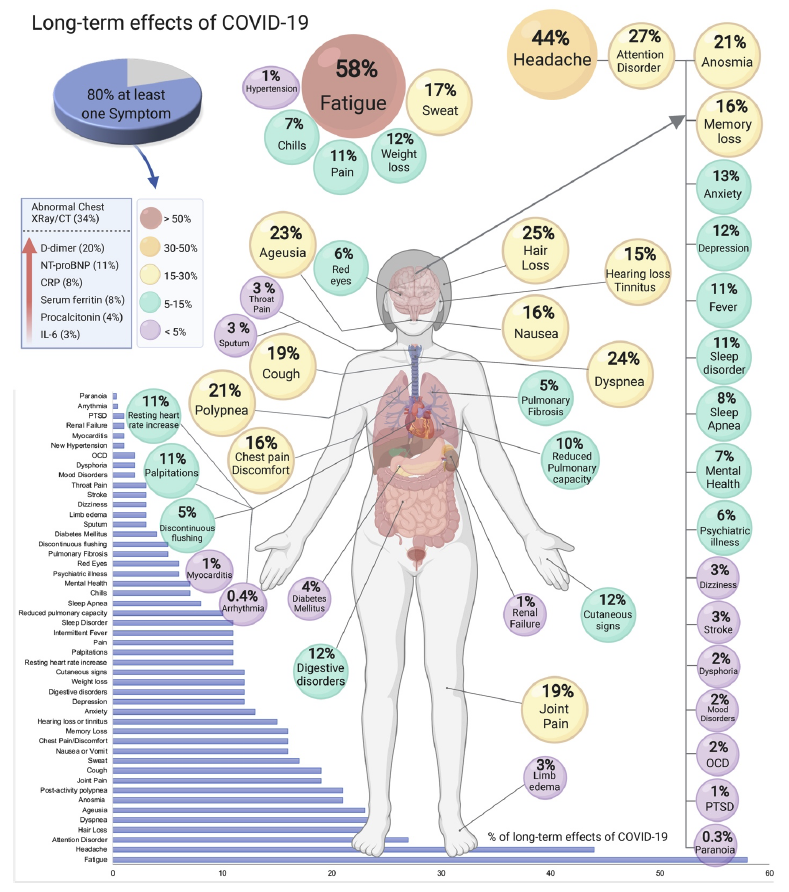
Source: medRxiv
Syndromes
As health care providers and scientists learn more, an early step taken to help better define PASC has been to group together the wide range of symptoms experienced by COVID-19 survivors into distinct syndromes. A syndrome is defined as a collection of symptoms that tend to occur together, and syndromes can be used to define a condition when there is no definitive diagnostic test; examples include chronic fatigue syndrome and irritable bowel syndrome.
To date, at least one syndrome has been defined under the PASC umbrella. Investigation into cases of children who were hospitalized with a hyperinflammatory syndrome led to the development of a case definition for what was named multisystem inflammatory syndrome in children, or MIS-C. People with this syndrome are under 21 years of age and have multiple organs affected and require hospitalization; some are critically ill. There is now recognition of a similar syndrome among adults, multisystem inflammatory syndrome in adults, or MIS-A. The incidence of both MIS-C and MIS-A are thought to be very low. From March 1 through March 10, 2020, the incidence of MIS-C in New York State among people younger than 21 years was 2 per 100,000 individuals, whereas the incidence of COVID-19 was 322 per 100,000 individuals. As of May 3, 2021, 3,742 cases of MIS-C had been reported in the United States. There is evidence that children may also experience symptoms of PASC that do not meet criteria for MIS-C.
Other sequelae of COVID-19 may overlap with previously defined syndromes or conditions that are not specific to COVID-19. For example, people who have been treated in an intensive care unit may develop post–intensive care syndrome (PICS). This syndrome is characterized by physical, cognitive and psychological symptoms that can have a profound and lasting effect on health. Patients with COVID-19 in the intensive care unit may be at greater risk of developing PICS than patients with other diagnoses in the intensive care unit; a risk factor for PICS is a type of serious lung injury (acute respiratory distress syndrome) which can be caused by COVID-19. Another example is chronic fatigue syndrome, also called myalgic encephalomyelitis, a syndrome characterized by exertional fatigue and other debilitating symptoms, which may overlap with health effects reported by those with PASC. Lasting respiratory symptoms may occur as sequelae of pneumonia caused by other pathogens – including respiratory viruses such as influenza and the coronaviruses that cause severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS – which can cause scarring of the lungs during acute infection.
Pathophysiology
While researchers are trying to better define PASC, they also are trying to understand what may be causing the symptoms. Many hypotheses on causation have been put forward. The two leading hypotheses are that symptoms arise from direct tissue damage due to the SARS-CoV-2 virus or from the immune response during acute infection. Another hypothesis is that health effects are caused by an ongoing dysregulated immune response. As discussed above, some experiences of COVID-19 survivors are due to clinical syndromes that have been previously described as sequelae of other diseases or treatments, such as intensive care.
How many people experience Post-Acute Sequelae of SARS-CoV-2 infection (PASC)?
Given the lack of a consensus definition for PASC, it is difficult to quantify what proportion of people go on to develop them. One frequently cited estimate is that one in 10 people infected with SARS-CoV-2 will go on to develop symptoms that last beyond three weeks; this estimate comes from the large UK COVID Symptom Study, in which people report ongoing symptoms via a smartphone application. However, estimates on how frequently those with COVID-19 develop PASC vary widely among studies. This is for several reasons, including differences between populations selected to enroll in different studies, how symptoms are defined and measured and the length of study follow up. At this time, there is no definitive estimate of the proportion of COVID-19 survivors who will go on to develop PASC. Here we review several studies that have assessed new or persistent symptoms or new diagnoses in order to understand the prevalence and characteristics of post-acute COVID-19 sequelae.
One approach to estimating the prevalence of symptoms related to PASC is to monitor a cohort (group of patients) who were diagnosed with COVID-19. Studies have enrolled cohorts of COVID-19 patients recruited from one or several medical centers. For example, among 143 patients hospitalized with COVID-19 at one Italian medical center in April and May 2020, 87% reported at least one symptom, and 44% reported worsened quality of life 60 days after the onset of COVID-19 symptoms. This study only included patients with severe acute illness and followed patients for a relatively short time, both of which may contribute to the high estimated prevalence. A recent systematic review estimated that 73% of patients hospitalized with COVID-19 had at least one symptom 60 days later.
Studies of patients who had less severe acute illness estimate a lower prevalence of PASC than studies of patients with more severe acute illness, though prevalence of sequelae may still be above 50%. For example, among 180 patients who tested positive for COVID-19 from April-June 2020 in the Faroe Islands, 53% reported at least one symptom after 125 days. Similarly, 53% of approximately 400 patients attending one clinic in Germany reported at least one symptom 6-7 months after acute infection. In these two studies, most cases were mild or moderate during the acute phase of illness, with only 3-4% of patients hospitalized. In a study that followed a population-based cohort (recruited from the community) of people who had been diagnosed with COVID-19 in Michigan, a smaller percentage, 35% of 593 adults, reported persistent symptoms 60 days or more after onset. In this study, people who reported severe acute symptoms were approximately twice as likely to report persistent symptoms compared to those who reported mild acute symptoms. Of note, studies suggest that the types of symptoms experienced may differ by illness severity and that symptoms may be more severe among patients who were hospitalized during acute infection than among those who were not. However, given differences in study design, including follow-up timelines and how symptoms are measured, it is difficult to draw conclusions about how the severity of acute illness relates to the type and severity of long-term symptoms.
A key limitation of the above studies is the lack of a comparison group of patients without COVID-19 to which these prevalence estimates can be compared. This may result in symptoms being attributed to COVID-19 even if there is not a causal relationship. Some patients may experience new-onset symptoms due to something other than COVID-19. People experiencing ongoing symptoms may also be more likely to participate in these studies, which could result in overestimation of the proportion of COVID-19 survivors who have post-acute sequelae. The pandemic also may have indirectly increased the prevalence of many symptoms. For example, the negative effects of the pandemic on mental health have been recognized; economic hardship can take a significant toll on physical and mental health; and people with chronic conditions who experienced lapses in care during the pandemic may experience worsening of their health.
The large population-based COVID-19 Infection Survey in the United Kingdom is an example of a study that included a comparison group of people without COVID-19, which helps us understand whether these post-acute symptoms are more common among COVID-19 survivors compared to the general public. Participants in this study were invited to receive repeated surveys and regular COVID-19 testing for up to one year. Among more than 20,000 study participants who tested positive for COVID-19 between April 2020 and March 2021, 13.7% continued to experience symptoms for at least 12 weeks after their positive test. This was eight times higher than the prevalence of symptoms reported by study participants who had not tested positive for COVID-19. Among people who were experiencing symptoms 12 weeks after their initial positive test, almost all (97%) had at least one symptom during the acute phase although only 8% were hospitalized. The percentage of study participants who reported specific symptoms 5 and 12 weeks after diagnosis are shown below. Fatigue, cough, headache and muscle pain were the most common symptoms reported after 12 weeks.
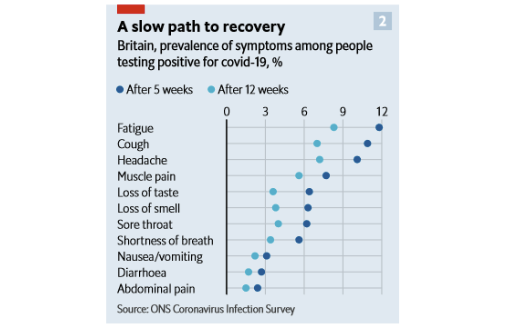
Source: Economist
Another approach to characterizing PASC is to analyze data from electronic health records. In the following three studies from the U.S., investigators leveraged data collected for health insurance claims to investigate a wide range of clinical sequelae among large numbers of patients and to compare between COVID-19 patients and patients with other diagnoses.
- A study of data from the Premier Healthcare Database (which includes data from more than 900 hospitals) found that 7% of 27,589 inpatients and 8% of 46,875 outpatients experienced post-COVID health conditions one to four months after their initial diagnosis. Post-COVID conditions included respiratory, circulatory and nervous system symptoms, as well as fatigue. Adult patients with a history of COVID-19 were more likely to experience these conditions compared to a control group of similar patients who did not have COVID-19. In contrast, children with COVID-19 (about 300 inpatients and 2,300 outpatients) did not experience a higher frequency of post-acute symptoms compared to a control group of children without COVID-19.
- Another large study used health insurance claims from UnitedHealth Group to investigate the occurrence of more than 50 clinical sequelae at least 21 days after COVID-19 diagnosis. Among more than 193,000 adults aged 18-65 with COVID-19, 14% developed at least one new type of sequelae that required medical care, compared to 9% of a control group of patients without COVID-19 and 13% of a second control group of patients with lower respiratory tract illnesses other than COVID-19. These findings serve as a reminder that other respiratory illnesses can also cause post-infectious sequelae and that the way a control group is selected can affect the findings of a comparison analysis. Although the risk of sequelae was higher among patients who were older, had comorbidities or were hospitalized with acute COVID-19, post-acute sequelae also occurred among patients younger than 50 without pre-existing conditions who did not have severe COVID-19.
- A larger number of post-COVID-19 conditions—more than 300—were explored in a study using data from the United States Department of Veterans Affairs. Investigators identified new medical diagnoses that were given between 30 days and six months after initial diagnosis of COVID-19 among more than 73,000 non-hospitalized and 13,000 hospitalized COVID-19 patients. They compared the incidence of new diagnoses in these groups to new diagnoses among control patients who did not have COVID-19 (4.9 million non-hospitalized control patients and nearly 14,000 patients hospitalized with seasonal influenza). COVID-19 patients were more likely to use health care resources and to experience a variety of respiratory, neurocognitive, mental health, metabolic, cardiovascular and gastrointestinal disorders in the six months after acute illness. Further, the likelihood of new diagnoses was greater among patients who had severe acute COVID-19; greater among hospitalized compared to non-hospitalized COVID-19 patients; and highest among COVID-19 patients treated in the intensive care unit.
These studies generally suggest that people who had COVID-19 experience more clinical sequelae than people without COVID-19, that more severe COVID-19 illness correlates with a higher likelihood of more disabling post-COVID-19 sequelae and that sequelae affect many different organ systems. These and other studies have also found that those who were hospitalized with COVID-19 were more likely to be readmitted or to die than people hospitalized for other reasons. In addition, these data highlight the potentially enormous burden of PASC on the health care system; a large number of people who had COVID-19 continued to seek care for months after their acute illness.
There are significant limitations to this study approach. First, some symptoms are not captured well in health insurance claims, and these sequelae may not be identified in this type of analysis. Second, diagnoses documented in health insurance claims may be influenced by insurer reimbursement policies. Third, people without regular access to health care are excluded from these studies. Fourth, these studies looked specifically for new-onset conditions starting 22 days or more after initial COVID-19 diagnosis, so they may have missed persistent symptoms that started at the same time as acute COVID-19.
Who is most at risk of developing Post-Acute Sequelae of SARS-CoV-2 infection (PASC)?
It is difficult to quantify and characterize risk factors for a condition that is not yet defined. It is likely that the risk factors for specific sequelae under the PASC umbrella vary, especially if different sequelae have different causes. Studies conducted to date suggest that four groups of people may be more likely to experience PASC: people with more severe acute illness, those who have underlying comorbidities, older adults and women.
Among studies that compared people with varying severities of acute illness—from asymptomatic to requiring intensive hospital care—many have found that more severe acute illness was associated with a greater number of post-acute sequelae. In a review of existing literature, the U.K. National Institute for Health Research found that among people hospitalized with COVID-19, 50-90% experienced symptoms at least two months after acute infection, whereas among people with COVID-19 who were not hospitalized, 20-30% of people experienced symptoms after one month and 10% experienced symptoms at least three months later. However, as noted in a recent editorial, hospitalized cases comprise only a small fraction of all COVID-19 patients. Therefore, the absolute number of people with PASC who were not hospitalized is likely to be larger than the absolute number of people with PASC who were hospitalized. This is reinforced by findings from a number of studies, such as a cohort study of patients from a clinic in Germany, which have described long-term symptoms among non-hospitalized COVID-19 survivors.
The evidence on other risk factors is less clear. In studies comparing people with more versus fewer comorbidities, it is not clear whether some post-acute sequelae could be exacerbations of pre-existing illness. Similarly, because people who are older tend to have more comorbidities and experience more severe COVID-19 illness, authors of a study on people who had COVID-19 in Michigan concluded that it was difficult to determine whether age is an independent risk factor for post-acute sequelae. Although women made up a larger proportion of long-COVID patients in studies in China and France, women may also be more likely to seek health care and report their symptoms than men.
Sources of potential significant bias
Many COVID-19 survivors were never diagnosed when they were acutely infected. In many parts of the world, including the U.S., access to testing was limited during the early stages of the pandemic and certain symptom or risk factor criteria had to be fulfilled to access testing. Even where specific criteria for testing have been relaxed, access may not be uniform. For example, in the U.S., disparities in testing by geography and race and ethnicity have been observed. In addition, those who did not experience symptoms during acute infection and were unaware they had COVID-19 may never have been tested. Those without a confirmed COVID-19 diagnosis may not be recognized as suffering from PASC or may be excluded from studies on PASC, potentially biasing study findings.
Addressing the impacts of Post-Acute Sequelae of SARS-CoV-2 infection (PASC)
For those with long-lasting symptoms, it is critical to better describe PASC and its causes so that we can provide effective care and support. Given the spectrum of health effects that may occur, people with PASC may have diverse needs. Some may recover with holistic support, rest, treatment targeted to specific symptoms (e.g. medication for headaches) and gradual increase in activity. For others, a more intensive approach that involves specialists from a range of clinical disciplines may be needed. Patients with post-intensive care unit syndrome (PICS) may benefit from multidisciplinary specialist rehabilitation, and a similar approach may help some people with PASC whether or not they meet criteria for PICS as well. Multidisciplinary care models are being used in some centers that treat COVID-19 survivors with lasting symptoms; such clinics now exist in at least 33 U.S. states. An improved understanding of the biological mechanisms that cause PASC may help identify targeted treatments for some patients. For example, those with fatigue may benefit from treatments used for patients with chronic fatigue syndrome. If some symptoms are caused by ongoing inflammation, anti-inflammatory drugs may be helpful. Novel treatments, rather than existing drugs used for other conditions, also may help. It has been reported that some people with PASC feel better after being vaccinated against COVID-19. A preprint analysis found that among 900 people who reported lasting symptoms after COVID-19, 57% reported an overall improvement in their symptoms after vaccination, 19% reported that their symptoms worsened and 25% reported no change. Researchers are working to better understand the effect of COVID-19 vaccination on PASC.
Persistent health effects after COVID-19 may have serious impacts on other aspects of wellness, including social function. A preprint analysis of data obtained through a survey distributed via social media found that among 3,762 respondents from 56 countries who had experienced symptoms consistent with COVID-19, 90% reported symptoms lasting beyond 90 days, 45% reported requiring a reduced work schedule compared to pre-illness and 22% reported not working for health reasons. Data from the United Kingdom suggest that 1.5% of the working age population has symptoms of PASC. Sequelae of COVID-19 may disproportionately affect the health and function of those who are especially vulnerable to health problems due to age or existing comorbidities, as well as those with employment, housing or financial insecurity. Some may have difficulty accessing care for their symptoms, including people who were not diagnosed when they were acutely infected with SARS-CoV-2, those who do not have relationships with care providers, those who encounter barriers to accessing health care and those who had mild acute infections and may be overlooked by providers. In addition, because of the wider effects that long-lasting symptoms can have, medical treatment is just one type of support people with PASC may need. New York City’s recently launched AfterCare program is one example of a city-wide initiative to provide accessible medical and social support for those who had COVID-19. This program reaches out to people who were in the New York City’s Test & Trace program and connects those dealing with long-term health and social effects with resources.
Conclusion
Considerable work is needed to better understand the Post-Acute Sequelae of SARS-CoV-2 infection (PASC). This should begin with defining the condition(s) and health effects it encompasses and determining what is causing those effects. This knowledge will support those experiencing ongoing symptoms and help researchers identify treatments to help people with PASC in their recovery. We know that COVID-19 is a complex disease that can have profound effects on nearly every part of the body during acute infection as well as over the longer term. We also know that if even a small proportion of those infected with SARS-CoV-2 globally go on to experience long-term symptoms, the societal impacts could be profound. We must develop a better understanding of how many people experience long-lasting symptoms so that medical, public health and social support systems may respond adequately. The importance of preventing the long-term health effects of COVID-19 cannot be overstated. Vaccines are highly effective in preventing both symptomatic and asymptomatic infection and thus offer excellent protection. This is just one of many reasons why universal access to COVID-19 vaccines should be a global priority.
FAQ: Did the COVID-19 vaccine work if you didn’t experience side effects?
After receiving a COVID-19 vaccine, some people experience adverse effects such as fatigue or headaches. These reactions can be a sign that the immune system is working. But what about people who do not experience any side effects? Does an absence of adverse effects mean the vaccine did not work?
Scientists distinguish two types of adverse effects after vaccines: local reactions that occur at the site of injection—pain, swelling or redness—and systemic reactions, or symptoms throughout the rest of the body, such as fatigue, fever or nausea. Both types may be caused by activation of the inflammatory immune system and typically subside within 1-3 days after vaccination.
Not everyone who receives a COVID-19 vaccine experiences side effects. During trials, about 20-30% of mRNA vaccine (Moderna, Pfizer) recipients and 40-55% of J&J (Janssen) vaccine recipients did not experience any systemic side effects after COVID-19 vaccination.The U.S. Centers for Disease Control and Prevention (CDC) summarized the proportion of people who reported local and systemic side effects during the trials for the Moderna, Pfizer, and J&J vaccines in adults:
- Across all three vaccines, the most common local side effect was pain at the injection site, which was reported by 33-58% of J&J trial participants and 65-90% of Pfizer and Moderna trial participants, as well as 10-20% of trial participants who received a placebo injection (an injection with saline instead of a vaccine).
- The most commonly reported systemic side effects were fatigue and headache, which were reported by as many as 30-45% of J&J vaccine recipients and 50-60% of mRNA vaccine recipients. Side effects were more commonly reported by recipients of the Moderna compared to the Pfizer vaccine.
- Fever was less commonly reported. Among older age groups in the vaccine trials, only 3% of J&J vaccine recipients and 10-11% of mRNA vaccine recipients reported having a fever. Among younger age groups, the percentage was slightly higher for all three vaccines at about 15-18% of vaccine recipients.
- Overall, about 80% of younger participants and 70% of older trial participants who received mRNA vaccines reported at least one systemic side effect. Only 62% of younger and 45% of older participants who received the J&J vaccine reported at least one systemic side effect.
Fatigue and headache were the most common systemic side effects reported during the first month of the U.S. vaccine rollout (Dec. 14, 2020-Jan. 13, 2021). Among 1.6 million people who participated in the CDC v-safe program after receiving an mRNA vaccine during this time, about one-third reported fatigue or headache, whereas only 11% of people reported experiencing fever.
Who is more likely to experience side effects after receiving a COVID-19 vaccine?
- During clinical trials, systemic side effects were more likely to be reported by younger people compared to older vaccine recipients.
- For the mRNA vaccines, systemic side effects were more likely to be reported after the second dose compared to the first dose.
- Data from more than 600,000 people in the United Kingdom also suggest that people with previous SARS-CoV-2 infection were between 1.5 to 3 times more likely to experience side effects after vaccination with the Pfizer or AstraZeneca vaccines compared to people who were not known to have been previously infected with the virus.
There are many different components of the immune system. Some parts cause the short-term side effects that some people experience after vaccination, while other parts, collectively known as the “adaptive immune response,” provide long-term protection against COVID-19. The adaptive immune response takes time to develop. This system includes antibodies and memory immune cells (memory B cells) that recognize a specific pathogen such as the SARS-CoV-2 virus. A small study of 44 people who received the Pfizer or Moderna COVID-19 vaccines found that people who experienced systemic side effects had on average slightly stronger antibody responses after the second dose than people who did not experience systemic side effects after vaccination. However, there was no difference in the memory B cell response between people who did and did not have systemic side effects. The authors concluded that further study was needed to clarify whether vaccine-induced side effects correlate with the strength of the adaptive immune response.
Although some people in the vaccine trials experienced no side effects, and the chance of experiencing side effects was correlated with age, data from vaccine trials and the real world show that all age groups are highly protected against COVID-19 by the Pfizer, Moderna and J&J vaccines.
For those who do experience side effects after the vaccine, the CDC has provided recommendations to minimize discomfort.
Research Highlights
Excess deaths associated with covid-19 pandemic in 2020: age and sex disaggregated time series analysis in 29 high income countries
(BMJ, May 2021)
- The 29 countries included were OECD countries that had available weekly mortality data by age and sex from 2016 to 2020. Projected deaths for 2020 were modeled based on historical weekly mortality rates from 2016-2019 by age and sex for each country.
- For countries with excess deaths (all but Norway, Denmark and New Zealand), excess death rates increased exponentially by age.
- The overall pandemic peaks in the 29 countries were March through May and October through December. The trajectory of excess deaths varied across countries, with some experiencing both peaks, some only one and some (particularly those with few excess deaths) experiencing no real difference across the year.
- In most countries, excess deaths exceeded reported deaths due to COVID-19. For instance, excess deaths were 30% higher in the U.K. and the U.S. and more than 50% higher in Spain, Lithuania and Poland. However, in a handful of countries—New Zealand, Norway, Denmark, Israel, France, Germany, Belgium and Switzerland—reported COVID-19 deaths were actually higher than excess deaths, due to lower than expected deaths from other causes.
- This study shows the likely toll of COVID-19 in 2020 and describes how it varied widely across high-income countries. Excess deaths that are not reported as COVID-19 are likely a combination of COVID-19 deaths that were misclassified and deaths due to other causes that were exacerbated by conditions of the pandemic (e.g., less access to care for any cause). While it is not possible to know the true number of deaths that would have taken place in 2020 had COVID-19 not happened, the detailed modeling approach taken in this study provides a good estimation.

Patient care and clinical outcomes for patients with COVID-19 infection admitted to African high-care or intensive care units (ACCCOS): a multicentre, prospective, observational cohort study
(Lancet, May)
- This prospective observational cohort study was conducted among adults ages 18 years or older who were admitted with suspected or confirmed COVID-19 to intensive care units in 64 hospitals in ten African countries. For the purposes of comparing this cohort to a global cohort of critically ill COVID-19 patients, the authors also conducted a meta-analysis of literature on COVID-19 critical care outcomes.
- From May to December 2020, 6,779 patients were referred to critical care. Of these, 3,752 (55%) were admitted to intensive care and 3,140 patients participated in the study. Among 3,140 participants, 2,995 (95%) were confirmed to have COVID-19 and the mean age was 56 years. Sequential organ failure assessment scores upon referral or admission suggested that the African cohort was not as sick as the global cohort.
- In-hospital mortality within 30 days of admission was 48% (1,483 of 3,077 patients with outcome data). The overall reported global mortality was 32%, which translates to an excess mortality of 11 to 23 deaths per 100 patients in the Africa cohort compared with the global average. Patients who received mechanical ventilation on admission had a mortality rate of 80% (918 of 1,164 patients).
- Most of the hospitals were government-funded (88%), and most were tertiary level (70%) hospitals. Among 57 hospitals with available data: 27 (47%) had oxygen available from vacuum insulated evaporators; the median surge ventilator capacity was five; 49 (86%) sites could provide pulse oximetry to all patients; and 39 (68%) could perform dialysis. The average number of intensive care doctors was two per site, and the daytime doctor:patient ratio was 1:4 while the nurse:patient ratio was 1:2.
- A number of factors were independently associated with mortality, including age (OR per year 1.03; 95% CI 1.02 – 1.04); HIV (1.91; 1.31 – 2.79) and a range of other comorbidities; delay in admission to ICU after arrival (2.14; 1.42 – 3.22); a need for high-flow oxygen (2.72; 1.46 – 5.08); and a need for mechanical ventilation (15.27; 8.51 – 27.37). Steroid therapy was associated with survival (0.55; 0.37 – 0.81). One in two patients died without receiving oxygen.
- The authors commented that physical resource constraints are a major contributor to high mortality rates and though it appears that human resources are not, a lack of human resources likely contributed to low rates of intensive care unit admission and to admission delays, and thus also contributed to mortality.
- Limitations include that data came primarily from tertiary care hospitals; it is likely that mortality among critically ill patients with COVID-19 is even higher at non-tertiary centers than these data suggest.
Suggested citation: Cash-Goldwasser S, Jones SA, Bochner A, Cobb L and Frieden TR. In-Depth COVID-19 Science Review June 4, 2021. Resolve to Save Lives. 2021 June 4. Available from https://preventepidemics.org/covid19/science/review/





