Abstract
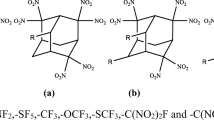
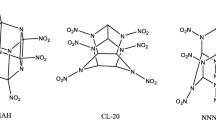
Wiberg bond index and natural bond orbital analyses were used to examine the effect of strain on trigger bonds for a series of organic-cage molecules. In substituted cage hydrocarbons, weakening of the interior C–C bonds and strengthening of exterior C–X bonds are explained in terms of the contributions of the carbon 2s and 2p atomic orbitals to the bonds. The interior C–C, rather than C–NO2, bonds are expected to break to initiate explosive decomposition in nitro-substituted tetrahedranes, prismanes, and cubanes. Activation of C–C bonds in cage molecules increases with nitro substitution, but persubstituted cages are not necessarily the most activated. For example, heptanitrocubane is predicted to be more sensitive than octanitrocubane, consistent with experimental studies. In contrast, for a series of hypothetical nitroester substituted prismanes, the strengthening of the exterior C–ONO2 bond weakens the O–NO2 bond more than the interior C–C bonds. In CL-20 and TEX, cage strain as determined by WBI analysis in the fused five- and six-membered rings is less significant than for the cage hydrocarbon. In these known energetic materials, the N–NO2 trigger bonds are activated by the orientation of the nitro groups.






Similar content being viewed by others
References
Talawar MB, Sivabalan R, Mukundan T et al (2009) Environmentally compatible next generation green energetic materials (GEMs). J Haz Mat 161:589–607. https://doi.org/10.1016/j.jhazmat.2008.04.011
Giles J (2004) Green explosives: collateral damage. Nature 427:580–581. https://doi.org/10.1038/427580a
Witze A (2013) Green fuels blast off. Nature 500:509–510. https://doi.org/10.1038/500509a
Yin P, Zhang Q, Shreeve JM (2016) Dancing with energetic nitrogen atoms: versatile N-functionalization strategies for N-heterocyclic frameworks in high energy density materials. Acc Chem Res 49:4–16. https://doi.org/10.1021/acs.accounts.5b00477
Kamlet M, Adolph H (1979) Relationship of impact sensitivity with structure of organic high explosives. 2. Polynitroaromatic explosives. Propellants Explos 4:30–34. https://doi.org/10.1002/prep.19790040204
Sikder AK, Maddala G, Agrawal JP, Singh H (2001) Important aspects of behaviour of organic energetic compounds: a review. J Haz Mat 84:1–26. https://doi.org/10.1016/S0304-3894(01)00178-9
Novak I (2003) Substituent effects on steric strain. Chem Phys Lett 380:258–262. https://doi.org/10.1016/j.cplett.2003.08.109
Chi W-J, Li L-L, Li B-T, Wu H-S (2012) Density functional calculations for a high energy density compound of formula C6H6−n(NO2)n. J Mol Model 18:3695–3704. https://doi.org/10.1007/s00894-012-1367-6
Chi W-J, Li L-L, Li B-T, Wu H-S (2013) Theoretical investigation on detonation performances and thermodynamic stabilities of the prismane derivatives. J Mol Model 19:1049–1057. https://doi.org/10.1007/s00894-012-1648-0
Zhang M-X, Eaton PE, Gilardi R (2000) Hepta- and octanitrocubanes. Angew Chem Int Ed 39:401–404. https://doi.org/10.1002/(SICI)1521-3773(20000117)39:2%3c401:AID-ANIE401%3e3.0.CO;2-P
Eaton PE, Zhang M-X, Gilardi R et al (2002) Octanitrocubane: a new nitrocarbon. Propellants Explos Pyrotech 27:1–6. https://doi.org/10.1002/1521-4087(200203)27:1%3c1:AID-PREP1%3e3.0.CO;2-6
Politzer P, Seminario JM (1989) Computational determination of the structures and some properties of tetrahedrane, prismane, and some of their aza analogs. J Phys Chem 93:588–592. https://doi.org/10.1021/j100339a019
Zhou G, Zhang J-L, Wong N-B, Tian A (2004) Computational studies on a kind of novel energetic materials tetrahedrane and nitro derivatives. J Mol Struct Theochem 668:189–195. https://doi.org/10.1016/j.theochem.2003.10.054
Politzer P, Seminario JM (1989) Computational analysis of the structures, bond properties, and electrostatic potentials of some nitrotetrahedranes and nitroazatetrahedranes. J Phys Chem 93:4742–4745. https://doi.org/10.1021/j100349a013
Rayne S, Forest K (2011) A G4MP2 and G4 theoretical study into the thermochemical properties of explosophore substituted tetrahedranes and cubanes. Propellants Explos Pyrotech 36:410–415. https://doi.org/10.1002/prep.201000165
Joshi KA, Gejji SP (2005) Molecular electrostatic potentials and electron densities in nitrotriprismanes. J Mol Struct Theochem 724:87–93. https://doi.org/10.1016/j.theochem.2005.02.050
Chi W-J, Li Z-S (2015) Molecular design of prismane-based potential energetic materials with high detonation performance and low impact sensitivity. C R Chim 18:1270–1276. https://doi.org/10.1016/j.crci.2015.06.018
Murray JS, Seminario JM, Politzer P (1991) Effects of the simultaneous presence of nitro and amine substituents in cubane and some azacubanes. Struct Chem 2:153–166. https://doi.org/10.1007/BF00676627
Kortus J, Pederson MR, Richardson SL (2000) Density functional-based prediction of the electronic, structural, and vibrational properties of the energetic molecule: octanitrocubane. Chem Phys Lett 322:224–230. https://doi.org/10.1016/S0009-2614(00)00425-5
Gejji SP, Patil UN, Dhumal NR (2004) Molecular electrostatic potentials and electron densities in nitrocubanes C8H8−α(NO2)α (α = 1–8): ab initio and density functional study. J Mol Struct THOECHEM 681:117–127. https://doi.org/10.1016/j.theochem.2004.05.012
Li J, Huang H, Huang Y, Dong H (2008) A theoretical study of highly nitrated azacubanes. J Energ Mat 26:230–245. https://doi.org/10.1080/07370650802182575
Richard RM, Ball DW (2009) Density functional calculations on the thermodynamic properties of a series of nitrosocubanes having the formula C8H8−x(NO)x (x = 1−8). J Haz Mat 164:1552–1555. https://doi.org/10.1016/j.jhazmat.2008.08.057
Li J (2009) An evaluation of nitro derivatives of cubane using ab initio and density functional theories. Theor Chem Acc 122:101–106. https://doi.org/10.1007/s00214-008-0489-5
Chaban VV, Prezhdo OV (2016) Energy storage in cubane derivatives and their real-time decomposition: computational molecular dynamics and thermodynamics. ACS Energy Lett 1:189–194. https://doi.org/10.1021/acsenergylett.6b00075
Kiselev VG, Goldsmith CF (2019) Accurate prediction of bond dissociation energies and barrier heights for high-energy caged nitro and nitroamino compounds using a coupled cluster theory. J Phys Chem A 123:4883–4890. https://doi.org/10.1021/acs.jpca.9b01506
Hrovat DA, Borden WT, Eaton PE, Kahr B (2001) A computational study of the interactions among the nitro groups in octanitrocubane. J Am Chem Soc 123:1289–1293. https://doi.org/10.1021/ja001636t
Zhou G, Wang J, He W-D et al (2002) Theoretical investigation of four conformations of HNIW by B3LYP method. J Mol Struct THEOCHEM 589–590:273–280. https://doi.org/10.1016/S0166-1280(02)00282-8
Okovytyy S, Kholod Y, Qasim M et al (2005) The mechanism of unimolecular decomposition of 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane. A computational DFT study. J Phys Chem A 109:2964–2970. https://doi.org/10.1021/jp045292v
Kholod Y, Okovytyy S, Kuramshina G et al (2007) An analysis of stable forms of CL-20: A DFT study of conformational transitions, infrared and Raman spectra. J Mol Struct 843:14–25. https://doi.org/10.1016/j.molstruc.2006.12.031
Tan B, Long X, Li J (2012) The cage strain energies of high-energy compounds. Comput Theor Chem 993:66–72. https://doi.org/10.1016/j.comptc.2012.05.033
Ghule VD, Jadhav PM, Patil RS et al (2010) Quantum-chemical studies on hexaazaisowurtzitanes. J Phys Chem A 114:498–503. https://doi.org/10.1021/jp9071839
Yang J, Wang G, Gong X et al (2018) High-energy nitramine explosives: a design strategy from linear to cyclic to caged molecules. ACS Omega 3:9739–9745. https://doi.org/10.1021/acsomega.8b00614
Koch E-C (2015) TEX—4,10-dinitro-2,6,8,12-tetraoxa-4,10-diazatetracyclo[5.5.0.05,9.03,11]-dodecane—review of a promising high density insensitive energetic material. Propellants Explos Pyrotech 40:374–387. https://doi.org/10.1002/prep.201400195
Pan Y, Zhu W, Xiao H (2017) Theoretical studies of a series of azaoxaisowurtzitane cage compounds with high explosive performance and low sensitivity. Comput Theor Chem 1114:77–86. https://doi.org/10.1016/j.comptc.2017.05.021
Isayev O, Gorb L, Qasim M, Leszczynski J (2008) Ab initio molecular dynamics study on the initial chemical events in nitramines: thermal decomposition of CL-20. J Phys Chem B 112:11005–11013. https://doi.org/10.1021/jp804765m
Kumar MA, Ashutosh P, Vargeese AA (2019) Decomposition mechanism of hexanitrohexaazaisowurtzitane (CL-20) by coupled computational and experimental study. J Phys Chem A 123:4014–4020. https://doi.org/10.1021/acs.jpca.9b01197
Molt RW, Bartlett RJ, Watson T, Bazanté AP (2012) Conformers of CL-20 explosive and ab initio refinement using perturbation theory: implications to detonation mechanisms. J Phys Chem A 116:12129–12135. https://doi.org/10.1021/jp305443h
Oliveira MAS, Borges I (2019) On the molecular origin of the sensitivity to impact of cyclic nitramines. Int J Quantum Chem 119:e25868. https://doi.org/10.1002/qua.25868
Ghosh M, Banerjee S, Khan MAS et al (2016) Understanding metastable phase transformation during crystallization of RDX, HMX and CL-20: experimental and DFT studies. Phys Chem Chem Phys 18:23554–23571. https://doi.org/10.1039/C6CP02185A
Li X-H, Zhang X-Z (2013) Computational studies on a high density cage compound hexanitrohexaazaisowurtzitane derivative. Can J Chem 91:369–374. https://doi.org/10.1139/cjc-2012-0468
Ju X-H, Wang Z-Y (2009) Prediction of caged polyaza polynitroamine (Tetracyclo-HMX) as energetic compound. J Energ Mat 27:133–143. https://doi.org/10.1080/07370650802405299
Wu Q, Zhu W, Xiao H (2013) Computer-aided design of two novel and super-high energy cage explosives: dodecanitrohexaprismane and hexanitrohexaazaprismane. RSC Adv 4:3789–3797. https://doi.org/10.1039/C3RA46625F
Qiu W, Ren F, Shi W, Wang Y (2015) A theoretical study on the strength of the C–NOt bond and ring strain upon the formation of the intermolecular H-bonding interaction between HF and nitro group in nitrocyclopropane, nitrocyclobutane, nitrocyclopentane or nitrocyclohexane. J Mol Model 21:114. https://doi.org/10.1007/s00894-015-2653-x
Wang B, Ren F, Shi W (2015) A theoretical investigation into the strength of N–NO2 bonds, ring strain and electrostatic potential upon formation of intermolecular H-bonds between HF and the nitro group in nitrogen heterocyclic rings CnH2nN–NO2 (n = 2–5), RDX and HMX. J Mol Model 21:302. https://doi.org/10.1007/s00894-015-2842-7
Du M, Wang X, Guo Z (2016) Theoretical design of bicyclo[2.2.1]heptane derivatives for high-energy density compounds with low impact sensitivity. Comput Theor Chem 1095:54–64. https://doi.org/10.1016/j.comptc.2016.09.008
Ren F, Cao D, Shi W, Gao H (2016) A theoretical prediction of the relationships between the impact sensitivity and electrostatic potential in strained cyclic explosive and application to H-bonded complex of nitrocyclohydrocarbon. J Mol Model 22:97. https://doi.org/10.1007/s00894-016-2967-3
Xiang D, Chen H, Zhu W, Xiao H (2016) Searching for a new family of high energy explosives by introducing N atoms, N-oxides, and NO2 into a cage adamantane. Can J Chem 94:667–673. https://doi.org/10.1139/cjc-2016-0174
Borges I (2008) Conformations and charge distributions of diazocyclopropanes. Int J Quantum Chem 108:2615–2622. https://doi.org/10.1002/qua.21671
Politzer P, Lane P, Murray JS (2011) Computational characterization of a potential energetic compound: 1,3,5,7-tetranitro-2,4,6,8-tetraazacubane. Cent Eur J Energy Mater 8:39–52
Wiberg KB (1968) Application of the pople-santry-segal CNDO method to the cyclopropylcarbinyl and cyclobutyl cation and to bicyclobutane. Tetrahedron 24:1083–1096. https://doi.org/10.1016/0040-4020(68)88057-3
Harper LK, Shoaf AL, Bayse CA (2015) Predicting trigger bonds in explosive materials through Wiberg bond index analysis. ChemPhysChem 16:3886–3892. https://doi.org/10.1002/cphc.201500773
Shoaf AL, Bayse CA (2018) Trigger bond analysis of nitroaromatic energetic materials using Wiberg bond indices. J Comput Chem 39:1236–1248. https://doi.org/10.1002/jcc.25186
Shoaf AL, Bayse CA (2019) The effect of nitro groups on N2 extrusion from aromatic azide-based energetic materials. New J Chem 43:15326–15334. https://doi.org/10.1039/C9NJ03220G
Cao Q (2013) Dinitroamino benzene derivatives: a class new potential high energy density compounds. J Mol Model 19:2205–2210. https://doi.org/10.1007/s00894-013-1769-0
Gao H, Zhang S, Ren F et al (2015) Theoretical insight into the co-crystal explosive of 2,4,6,8,10,12-hexanitrohexaazaisowurtzitane (CL-20)/1,1-diamino-2,2-dinitroethylene (FOX-7). Comput Mater Sci 107:33–41. https://doi.org/10.1016/j.commatsci.2015.05.009
Li X-H, Zhang R-Z, Zhang X-Z (2013) Theoretical investigations on heat of formation, detonation performance, and pyrolysis mechanism of 1,3,5,7,9,11-hexo(nitramine)-2,4,6,8,10,12- hexaazatetracyclo[5,5,0,0,0]dodecane. Can J Chem 91:1213–1218. https://doi.org/10.1139/cjc-2013-0321
Jeong K (2018) New theoretically predicted RDX- and -HMX-based high-energy-density molecules. Int J Quantum Chem 118:e25528. https://doi.org/10.1002/qua.25528
Foster JP, Weinhold F (1980) Natural hybrid orbitals. J Am Chem Soc 102:7211–7218. https://doi.org/10.1021/ja00544a007
Reed A, Weinhold F (1983) Natural bond orbital analysis of near-Hartree–Fock water dimer. J Chem Phys 78:4066–4073. https://doi.org/10.1063/1.445134
(2009) Gaussian 09. Gaussian, Inc., Wallingford, CT
Dunning TH (1971) Gaussian basis functions for use in molecular calculations. III. Contraction of (10s6p) atomic basis sets for the first-row atoms. J Chem Phys 55:716–723. https://doi.org/10.1063/1.1676139
Weigend F, Ahlrichs R (2005) Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys Chem Chem Phys 7:3297–3305. https://doi.org/10.1039/B508541A
Glendening E, Reed AE, Carpenter J, Weinhold F NBO Version 3.1
Politzer P, Domelsmith LN, Abrahmsen L (1984) Electrostatic potentials of strained systems: cubane, homocubane, and bishomocubane. J Phys Chem 88:1752–1758. https://doi.org/10.1021/j150653a018
Owens FJ (1999) Molecular orbital calculation of decomposition pathways of nitrocubanes and nitroazacubanes. J Mol Struct Theochem 460:137–140. https://doi.org/10.1016/S0166-1280(98)00312-1
Zhang J, Xiao H (2002) Computational studies on the infrared vibrational spectra, thermodynamic properties, detonation properties, and pyrolysis mechanism of octanitrocubane. J Chem Phys 116:10674–10683. https://doi.org/10.1063/1.1479136
Acknowledgements
M.J. was supported through an NSF-REU site (CHE-1659476). Calculations were performed on the Turing High Performance Cluster maintained by ODU Information Technology Services.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bayse, C.A., Jaffar, M. Bonding analysis of the effect of strain on trigger bonds in organic-cage energetic materials. Theor Chem Acc 139, 95 (2020). https://doi.org/10.1007/s00214-020-02604-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-020-02604-0