Source Identification and Quantification of Seepage Water in a Coastal Mine, in China
Abstract
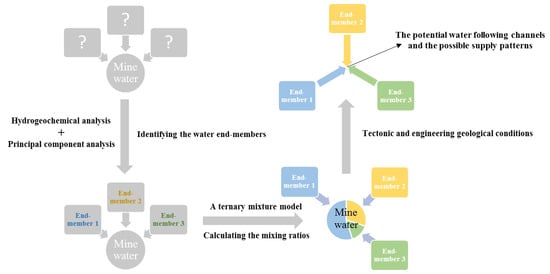
:1. Introduction
2. Study Area
2.1. Geological Setting
2.2. Hydrogeological Conditions
3. Materials and Methods
3.1. Sampling and Analytical Procedures
3.2. Principal Component Analysis (Based on Hydrogeochemical Analysis)
3.3. Mixing Calculation
3.4. Deviation Analysis
4. Results and Discussion
4.1. Hydrochemical Analysis
4.2. Principal Component Analysis (Determination of End-Members)
4.3. Mixing Calculation and Deviation Analysis
4.4. Discussion: The Potential Water Flow Channel
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rona, P.A. Resources of the sea floor. Science 2003, 299, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Commeau, R.F.; Clark, A.; Johnson, C.; Manheim, F.T.; Aruscavage, P.J.; Lane, C.M. Ferromanganese crust resources in the Pacific and Atlantic Oceans. In Proceedings of the Oceans Conference (IEEE), Washington, DC, USA, 10–12 September 1984; p. 421. [Google Scholar]
- Chung, J.S. Deep-ocean mining: Technologies for manganese nodules and crusts. Int. J. Offshore Polar Eng. 1996, 6, 1–11. [Google Scholar]
- Li, X.B.; Li, D.Y.; Liu, Z.X.; Zhao, G.Y.; Wang, W.H. Determination of the minimum thickness of crown pillar for safe exploitation of a subsea gold mine based on numerical modelling. J. Rock Mech. Min Sci. 2013, 57, 42–56. [Google Scholar] [CrossRef]
- Sui, W.H.; Xu, Z.M. Risk assessment for coal mining under sea area. New Front. Eng. Geol. Environ. 2013, 9, 199–202. [Google Scholar]
- Liu, Z.X.; Dang, W.G.; He, X.Q. Undersea safety mining of the large gold deposit in Xinli District of Sanshandao Gold Mine. Int. J. Min. Met. Mater. 2012, 19, 574–583. [Google Scholar] [CrossRef]
- Ma, F.S.; Zhao, H.J.; Guo, J. Investigating the characteristics of mine water in a subsea mine using groundwater geochemistry and stable isotopes. Environ. Earth Sci. 2015, 74, 6703–6715. [Google Scholar] [CrossRef]
- Alfarrah, N.; Walraevens, K. Groundwater overexploitation and seawater intrusion in coastal areas of arid and semi-arid regions. Water 2018, 10, 143. [Google Scholar] [CrossRef]
- Mauryaa, P.; Kumaria, R.; Mukherjeeb, S. Hydrochemistry in integration with stable isotopes (δ18O and δD) to assess seawater intrusion in coastal aquifers of Kachchh district, Gujarat, India. J. Geochem. Explor. 2019, 196, 42–56. [Google Scholar] [CrossRef]
- Ma, F.S.; Yang, Y.S.; Yuan, R.M.; Cai, Z.; Pan, S. Study of shallow groundwater quality evolution under saline intrusion with environmental isotopes and geochemistry. Environ. Geol. 2007, 51, 1009–1017. [Google Scholar] [CrossRef]
- Guo, J.; Zhao, H.J.; Ma, F.S.; Li, K.P.; Zhao, C.H. Investigating the permeability of fractured rock masses and the origin of water in a mine tunnel in Shandong province, China. Water Sci. Technol. 2015, 72, 2006–2017. [Google Scholar] [CrossRef]
- Li, P.; Wu, J.; Qian, H. Preliminary assessment of hydraulic connectivity between river water and shallow groundwater and estimation of their transfer rate during dry season in the Shidi River, China. Environ. Earth Sci. 2016, 75, 99. [Google Scholar] [CrossRef]
- Liu, C.; Peng, B.; Qin, J. Geological Analysis and Numerical Modeling of Mine Discharges for the Sanshandao Gold Mine in Shandong, China: 1. Geological Analysis. Mine Water Environ. 2007, 26, 160–165. [Google Scholar] [CrossRef]
- Fan, Y.T.; Chen, Y.N.; He, Q.; Li, W.H.; Wang, Y. Isotopic Characterization of River Waters and Water Source Identification in an Inland River, Central Asia. Water 2016, 8, 286. [Google Scholar] [CrossRef]
- Gu, H.Y.; Ma, F.S.; Guo, J.; Li, K.P.; Lu, R. Assessment of Water Sources and Mixing of Groundwater in a Coastal Mine: The Sanshandao Gold Mine, China. Mine Water Environ. 2018, 37, 351–365. [Google Scholar] [CrossRef]
- Gu, H.Y.; Ma, F.S.; Guo, J.; Li, K.P.; Lu, R. Hydrochemistry, multidimensional statistics, and rock mechanics investigations for Sanshandao Gold Mine, China. Arab. J. Geosci. 2017, 10, 62. [Google Scholar] [CrossRef]
- Peng, K.; Li, X.B.; Wang, Z.W. Hydrochemical characteristics of groundwater movement and evolution in the Xinli deposit of the Sanshandao gold mine using FCM and PCA methods. Environ. Earth Sci. 2015, 73, 7873–7888. [Google Scholar] [CrossRef]
- Long, A.J.; Valder, J.F. Multivariate analyses with end-member mixing to characterize groundwater flow: Wind Cave and associated aquifers. J. Hydrol. 2011, 409, 315–327. [Google Scholar] [CrossRef]
- Valder, J.F.; Long, A.J.; Davis, A.D.; Kenner, S.J. Multivariate statistical approach to estimate mixing proportions for unknown end members. J. Hydrol. 2012, 460–461, 65–76. [Google Scholar] [CrossRef]
- Semar, A.; Saibi, H. Multiparametric cartographic assessment of the hydrochemical groundwater of the Soummam Valley (Kabylia, Algeria). Environ. Prog. Sustain. Energy 2014, 33, 1357–1365. [Google Scholar]
- Shrestha, S.; Kazama, F. Assessment of surface water quality using multivariate statistical techniques: A case study of the Fuji river basin, Japan. Environ. Model. Softw. 2007, 22, 464–475. [Google Scholar] [CrossRef]
- Carrera, J.; Vázquez-Suñé, E.; Castillo, O.; Sánchez-Vila, X. A methodology to compute mixing ratios with uncertain end-members. Water Resour. Res. 2004, 40, 3687–3696. [Google Scholar] [CrossRef]
- Sun, L.; Chen, S.; Gui, H. Source identification of inrush water based on groundwater hydrochemistry and statistical analysis. Water Pract. Technol. 2016, 11, 448–458. [Google Scholar] [CrossRef]
- Laaksoharju, M.; Gascoyne, M.; Gurban, I. Understanding groundwater chemistry using mixing models. Appl. Geochem. 2008, 23, 1921–1940. [Google Scholar] [CrossRef]
- Laaksoharju, M.; Skårman, C.; Skårman, E. Multivariate mixing and mass balance (M3) calculations, a new tool for decoding hydrogeochemical information. Appl. Geochem. 1999, 14, 861–871. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Huang, W. Hydrogeological and engineering features of F3 fracture zone in Sanshan island gold mine district and prevention of hazards. J. Eng. Geol. 1994, 2, 62–72. (In Chinese) [Google Scholar]
- Gao, S.; Zhang, J.J.; Sun, S.S.; Wang, Y.Z. Hydrogeological Characteristics of Gold Deoposit in North Sea Area of Sanshandao. Gold Sci. Technol. 2016, 24, 11–16. (In Chinese) [Google Scholar]
- Wen, B.J.; Fan, H.R.; Hu, F.F.; Liu, X.; Yang, K.F.; Sun, Z.F.; Sun, Z.F. Fluid evolution and ore genesis of the giant Sanshandao gold deposit, Jiaodong gold province, China: Constrains from geology, fluid inclusions and H–O–S–He–Ar isotopic compositions. J. Geochem. Explor. 2016, 171, 96–112. [Google Scholar] [CrossRef]
- Wang, S.F. Analysis of hydrogeology for deep mining in Sanshandao Gold Mine. Nonferrous Mines. 2001, 30, 9–12. (In Chinese) [Google Scholar]
- Ye, B.L.; Peng, E.S. Study on the conducting-water structure model in the Sanshandao gold deposit. J. Cent-South Inst. Min. Metall. 1994, 25, 146–150. (In Chinese) [Google Scholar]
- Gao, M.S.; Zheng, Y.M.; Liu, S.; Wang, S.T.; Kong, X.H.; Zhao, J.M.; Guo, F. Palaeogeographic Condition for Origin of Underground Brine in Southern Coast of Laizhou Bay, Bohai Sea. Geol. Rev. 2015, 61, 393–400. (In Chinese) [Google Scholar]
- Su, Q.; Yu, H.J.; Xu, X.Y.; Yao, J.; Jiang, X.Y. Hydrochemical Characteristics of Underground Brine in Littoral Plain South of Laizhou Bay. Adv. Mar. Sci. 2011, 29, 163–169. (In Chinese) [Google Scholar]
- Costello, A.B.; Osborne, J.W. Best practices in exploratory factor analysis: Four recommendations for getting the most from your analysis. Pract. Assess. Res. Eval. 2005, 10, 173–178. [Google Scholar]
- Jolliffe, I. Principal Component Analysis. Encyclopedia of Statistics in Behavioral Science; Wiley: New York, NY, USA, 2005. [Google Scholar]
- Li, G.Q.; Wang, X.Q.; Meng, Z.P.; Zhao, H.J. Seawater inrush assessment based on hydrochemical analysis enhanced by hierarchy clustering in an undersea goldmine pit, China. Environ. Earth Sci. 2014, 71, 4977–4987. [Google Scholar] [CrossRef]
- Duan, X.L.; Ma, F.S.; Zhao, H.J.; Guo, J.; Gu, H.Y.; Lu, R.; Liu, G.W. Determining mine water sources and mixing ratios affected by mining in a coastal gold mine, in China. Environ. Earth Sci. 2019, 78, 299. [Google Scholar] [CrossRef]
- Wu, J.C.; Xue, Y.Q.; Zhang, Z.H. Experimental study on the cation exchange between water and rock in aquifer in the process of sea water intrusion. J. Nanjing University (Nat. Sci). 1996, 32, 72–76. (In Chinese) [Google Scholar]
- Yang, L.Q.; Deng, J.; Wang, Z.L.; Zhang, L.; Guo, L.N.; Song, M.C.; Zheng, X.L. Mesozoic gold metallogenic system of the Jiaodong gold province, eastern China. Acta Petrol Sin. 2014, 30, 2447–2467. [Google Scholar]
- Pang, Z.H.; Kong, Y.L.; Li, J.; Tian, J. An Isotopic Geoindicator in the Hydrological Cycle. Proc. Earth Planet. Sci. 2017, 17, 534–537. [Google Scholar] [CrossRef]
- Gu, H.Y.; Ma, F.S.; Guo, J.; Zhao, H.J.; Lu, R.; Liu, G. A Spatial Mixing Model to Assess Groundwater Dynamics Affected by Mining in a Coastal Fractured Aquifer, China. Mine Water Environ. 2018, 37, 405–420. [Google Scholar] [CrossRef]









| Mine Water | Seawater | Brine (375-20) | Freshwater | Quaternary Water | |||
|---|---|---|---|---|---|---|---|
| Minimum | Maximum | Mean | |||||
| δ18O | −5.60 | −1.05 | −2.17 | −0.18 | −3.09 | −8.03 | −2.36 |
| δD | −43.25 | −2.83 | −17.85 | −5.18 | −21.60 | −57.82 | −24.00 |
| K+ | 35.50 | 370.00 | 234.11 | 362.50 | 220.00 | 11.80 | 362.50 |
| Na+ | 6225.00 | 18,062.50 | 10,585.96 | 9050.00 | 20,000.00 | 188.20 | 9750.00 |
| Ca2+ | 424.80 | 5090.20 | 1203.13 | 380.80 | 2116.20 | 138.30 | 320.60 |
| Mg2+ | 194.40 | 2259.90 | 1184.79 | 1125.10 | 3030.20 | 42.50 | 1093.50 |
| Cl− | 12,028.20 | 36,544.00 | 20,307.64 | 16,468.30 | 40,196.80 | 212.30 | 17,456.30 |
| SO42− | 1306.40 | 3554.20 | 2426.91 | 2439.90 | 3842.40 | 405.40 | 2228.60 |
| EC | 28,700.00 | 63,800.00 | 42,921.21 | 44,400.00 | 64,700.00 | 1760.00 | 46,100.00 |
| TH | 4833.90 | 16,568.20 | 7854.18 | 5584.50 | 17,764.20 | 520.40 | 5304.20 |
| TDS | 22,020.10 | 62,017.80 | 36,131.19 | 29,985.20 | 69,666.10 | 1261.20 | 31,388.40 |
| Variables | Principal Components | |
|---|---|---|
| PC1 | PC2 | |
| δ18O | 0.52 | 0.80 |
| δD | 0.45 | 0.81 |
| Na+ | 0.98 | −0.01 |
| Ca2++Ma2+ | 0.86 | −0.46 |
| Cl− | 0.98 | −0.14 |
| SO42− | 0.82 | 0.24 |
| EC | 0.94 | −0.01 |
| TH | 0.91 | −0.36 |
| TDS | 0.99 | −0.11 |
| Eigenvalue | 6.48 | 1.72 |
| Explained variance (%) | 71.95 | 19.15 |
| Cumulative % of variance | 71.95 | 91.11 |
| Significant loadings are in bold | ||
| Model | Deviation | δ18O | δD | Na+ | Ca2+ | Mg2+ | Cl− | SO42− | Ca2+ + Mg2+ |
|---|---|---|---|---|---|---|---|---|---|
| Only PCA | Max (Cc > Cm) | 1.25 | 4.76 | 0.34 | 0.49 | 0.62 | 0.24 | 0.35 | |
| Max (Cc < Cm) | −0.26 | −0.45 | −0.09 | −0.44 | −0.66 | −0.08 | −0.45 | ||
| Mean | 0.19 | 0.26 | 0.00 | −0.11 | 0.11 | 0.01 | −0.03 | ||
| PCA (based on hydrogeochemical analysis) | Max (Cc > Cm) | 0.69 | 2.65 | 0.30 | 0.22 | 0.78 | 0.15 | ||
| Max (Cc < Cm) | −0.43 | −0.25 | −0.09 | −0.08 | −0.32 | −0.23 | |||
| Mean | 0.04 | 0.11 | 0.00 | 0.00 | 0.03 | −0.04 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, X.; Ma, F.; Guo, J.; Zhao, H.; Gu, H.; Liu, S.; Sun, Q. Source Identification and Quantification of Seepage Water in a Coastal Mine, in China. Water 2019, 11, 1862. https://doi.org/10.3390/w11091862
Duan X, Ma F, Guo J, Zhao H, Gu H, Liu S, Sun Q. Source Identification and Quantification of Seepage Water in a Coastal Mine, in China. Water. 2019; 11(9):1862. https://doi.org/10.3390/w11091862
Chicago/Turabian StyleDuan, Xueliang, Fengshan Ma, Jie Guo, Haijun Zhao, Hongyu Gu, Shuaiqi Liu, and Qihao Sun. 2019. "Source Identification and Quantification of Seepage Water in a Coastal Mine, in China" Water 11, no. 9: 1862. https://doi.org/10.3390/w11091862