Electrochemical and Colorimetric Nanosensors for Detection of Heavy Metal Ions: A Review
Abstract
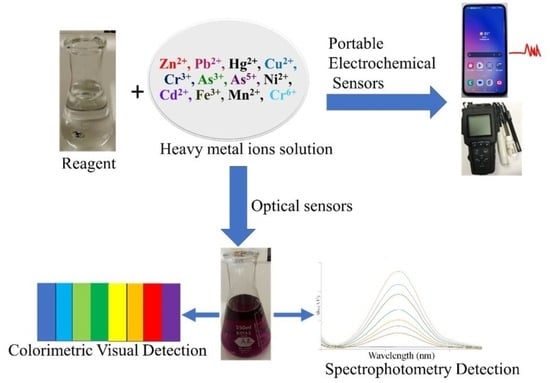
:1. Introduction and Overview
Conventional Heavy Metal Ion Analysis and Trace Element Detection Methods
2. Portable Electrochemical Nanosensors for the Detection of Heavy Metal Ions in Environmental Samples
2.1. Portable Electrochemical Nanosensors for the Detection of Heavy Metal Ions in Biological Samples
| Electrode Modification | Electrochemical Detection Method | Portability | Metal Ions | Linearity | LOD | Sample | Ref. |
|---|---|---|---|---|---|---|---|
| Phosphorus-doped biochar–attapulgite/bismuth film electrode decorated with magnetic Fe3O4 nanoparticles (MBA-BiFE) | SWASV | Smartphone-operated SPE | Cd(II) Pb(II) Hg(II) | 0.1 nM–5 μM, 0.01 nM–7 μM, 0.1 nM–3 μM | 0.036 nM 0.003 nM 0.011 nM | Tap water Lake water | [94] |
| Thymine acetic acid anchored with cysteamine-conjugated core-shell Fe3O4@Au nanoparticles (Fe3O4@Au/ CA/T-COOH) | DPASV | SPE/plastic chip sample holder | Hg(II) | 1–200 μg/L and 200–2200 μg/L | 0.5 μg/L | Wastewater | [95] |
| Butterfly-shaped silver nanostructure (AgNS) | DPASV | SPE | Cd(II) Pb(II) Cu(II) Hg(II) | 5–300 ppb 5–300 ppb 50–500 ppb 5–100 ppb | 0.4 ppb 2.5 ppb 7.3 ppb 0.7 ppb | Tap water Rainwater Lake water | [96] |
| Silver nanowires, hydroxymethyl propyl cellulose, chitosan, and urease (AgNWs/HPMC/CS/Urease) | CV | SPE | Hg(II) | 5–25 μM | 3.94 μM | Drinking water | [97] |
| Epitaxial Graphene on SiC | CSWASV | Portable in-house built potentiostat | CdCl2 CuSO4 HgCl2 PbCl2 | Spiked samples 100–3000 ppb | - | Sea water | [98] |
| Chitosan/PANi–Bi nanoparticle@graphene oxide multi-walled carbon nanotubes (CS/PANi–Bi NP@GO–MWCNT) | DPV | Portable device with an in situ signal analysis circuit, a Bluetooth chip, a photocured 3D-printed shell, and an electrode sleeve interface | Hg(II) Cu(II) | 10 ppb 0.998 ppm | Tap water | [99] | |
| Zirconium-based MOF material, UiO-66(Zr)–NH2 | DPASV | SPE | Pb(II) | 0.100–500 nM | 0.0492 ± 0.00523 nM | Tap water | [100] |
| Carbon fiber paper, CoMOF, AuNPs, and glutathione (CFP/CoMOF/AuNPs/GSH) | SWV | Carbon fiber paper electrode | Cd(II) | 0.001–1 μm | 1.0 nM | Lake water River water | [101] |
| Covalent organic framework (COFDATA-TP) | SWASV | SPE | Hg(II) Cu(II) Pb(II) Cd(II) | 0.0085–8.00 μM 0.015–8.00 μM 0.0056–8.00 μM 0.0069–8.00 μM | 2.80 nM 5.01 nM 1.83 nM 2.91 nM | River water | [102] |
| Silica isoporous membrane (SIM) | SWASV | SPE | Cd(II) Pb(II) Cu(II) Hg(II) | 0.2–20.0 µM 0.01–10.0 µM 0.2–20.0 µM 0.01–10.0 µM | 9.3 nM 1.1 nM 16.2 nM 1.4 nM | Soil | [103] |
| Chemically decorated mesoporous silica (SBA-15 and MCM-41) with L-cysteine (L-cys). | SWV | - | SBA-15 Cd(II) Pb(II) MCM-41 Cd(II) Pb(II) | 5–80 μg/L 10–80 μg/L 5–80 μg/L 10–80 μg/L | 0.22 μg/L 0.36 μg/L 0.23 μg/L 0.76 μg/L | Tap water Lake water | [104] |
| Ion-imprinted polymer film (IIP) | CV | SPE | Cd(II) | 10–1200 nM | 1.71 nM | Drinking water Tap water Marine water | [105] |
| Screen–printed gold working electrode with electroplated bismuth film (Bi/SPAuE) | SWASV | SPE | Pb(II) Cd(II) Zn(II) | 10–120 µg/L | 0.04 μg/L 0.02 μg/L 0.23 μg/L | Industrial wastewater | [106] |
| Hg/Bi-plated glassy carbon electrode | SWASV LSASV | - | Cd(II) Pb(II) As(III) | - | 0.03 μg/L 0.05 μg/L 0.15 μg/L | Tap water Mountain spring water River water | [107] |
| Metal | Normal Values * | |
|---|---|---|
| Blood | Urine | |
| As | <13 ng/mL (all ages) | 0–17 years: Not established > or =18 years: <24 g/g creatinine |
| Pb | 0–5 years: <3.5 g/dL > or =6 years: <5.0 g/dL Critical values Pediatrics (< or =15 years): > or =20.0 g/dL Adults (> or =16 years): > or =70.0 g/dL | 0–17 years: Not established > or =18 years: <0.6 g/g creatinine |
| Cd | <5.0 ng/mL (all ages) | 0–17 years: Not established > or =18 years: <2 g/g creatinine |
| Hg | <10 ng/mL (all ages) | 0–17 years: Not established > or =18 years: <2 g/g creatinine |
2.2. Portable Electrochemical Nanosensors for the Detection of Heavy Metal Ions in Food Samples
3. Colorimetric (UV-Visible) Nanosensors for Heavy Metal Ion Detections
3.1. Colorimetric Nanosensors for Detecting Heavy Metal Ions in Environmental Samples
3.1.1. Detection of Cu2+ Ions
3.1.2. Detection of Cr3+ and Cu2+ Ions
3.1.3. Detection of Fe3+, Cu2+, and Cr6+ Ions
3.1.4. Detection of Pb2+ Ions
3.1.5. Detection of Hg2+ Ions
3.1.6. Detection of Hg2+ and Pb2+ Ions
3.1.7. Detection of Hg2+ and Cd2+ Ions
3.1.8. Detection of Cd2+ and Ni2+ Ions
3.1.9. Detection of Various Heavy Metal Ions
3.2. Colorimetric Nanosensors for Detecting Heavy Metal Ions in Biological Samples
3.3. Detection of Heavy Metal Ions in Consumable Products
4. Conclusions and Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abeywickrama, C.J.; Wansapala, J. Review of organic and conventional agricultural products: Heavy metal availability, accumulation and safety. Int. J. Food Sci. Nutr. 2019, 4, 77–88. [Google Scholar]
- Vareda, J.P.; Valente, A.J.M.; Duraes, L. Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: A review. J. Environ. Manag. 2019, 246, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.I.; Daly, E.; Maggi, F. A review of ion and metal pollutants in urban green water infrastructures. Sci. Total Environ. 2014, 470–471, 695–706. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Namiesnik, J.; Rabajczyk, A. The speciation and physico-chemical forms of metals in surface waters and sediments. Chem. Speciat. Bioavailab. 2010, 22, 1–24. [Google Scholar] [CrossRef]
- Hussain, J.; Husain, I.; Arif, M.; Gupta, N. Studies on heavy metal contamination in Godavari river basin. Appl. Water Sci. 2017, 7, 4539–4548. [Google Scholar] [CrossRef]
- Joseph, L.; Jun, B.; Flora, J.R.V.; Park, C.M.; Yoon, Y. Removal of heavy metals from water sources in the developing world using low-cost materials: A review. Chemosphere 2019, 229, 142–159. [Google Scholar] [CrossRef]
- Mclaughlin, M.J.; Singh, B.R. Cadmium in soils and plants. Dev. Plant Soil Sci. 1999, 85, 257–267. [Google Scholar]
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A review on heavy metals contamination in soil: Effects, sources, and remediation techniques. Soil Sediment Contam. 2019, 28, 380–394. [Google Scholar] [CrossRef]
- Ayodele, O.S.; Madukwe, H.Y.; Adelodun, A.A. Geoenvironmental evaluation of toxic metals in the sediments of Araromi coastal area, Southwestern Nigeria. Environ. Qual. Manag. 2022, 31, 101–119. [Google Scholar] [CrossRef]
- Amoakwah, E.; Ahsan, S.; Rahman, M.A.; Asamoah, E.; Essumang, D.K.; Ali, M.; Islam, K.R. Assessment of heavy metal pollution of soil-water-vegetative ecosystems associated with artisanal gold mining. Soil Sediment Contam. Int. J. 2020, 29, 788–803. [Google Scholar] [CrossRef]
- Samanta, S.; Kumar, V.; Nag, S.K.; Saha, K.; Sajina, A.M.; Bhowmick, S.; Paul, S.K.; Das, B.K. Assessment of heavy metal contaminations in water and sediment of River Godavari, India. Aquat. Ecosyst. Health Manag. 2021, 24, 23–33. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.P.; Song, Z. Heavy metal contamination and ecological risk assessments in urban mangrove sediments in Zhanjiang Bay, South China. ACS Omega 2022, 7, 21306–21316. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, M.S.; Haider, S.M.B.; Meraj, G.; Bakar, M.A.; Islam, M.T.; Kunda, M.; Siddique, M.A.B.; Ali, M.M.; Mustary, S.; Mojumder, I.A.; et al. Assessment of heavy metal contamination in beach sediments of Eastern St. Martin’s Island, Bangladesh: Implications for environmental and human health risks. Water 2023, 15, 2494. [Google Scholar] [CrossRef]
- Köse, E.; Emiroğlu, Ö.; Çiçek, A.; Aksu, S.; Başkurt, S.; Tokatli, C.; Şahin, M.; Alper Uğurluoğlu, A. Assessment of ecologic quality in terms of heavy metal concentrations in sediment and fish on Sakarya River and Dam Lakes, Turkey. Soil Sediment Contam. 2020, 29, 292–303. [Google Scholar] [CrossRef]
- Januar, H.I.; Dwiyitno; Hidayah, I. Seasonal variation of heavy metal accumulation in environment and fishes from the Cirebon coast, Indonesia. Aquat. Ecosyst. Health Manag. 2021, 24, 121–129. [Google Scholar] [CrossRef]
- Parmar, D.K.; Khatkar, A.; Kumar, P.; Kumar, P.; Sharma, M.; Butail, N.P. Spatial variation of heavy metal contamination in roadside soil from hilly terrain of the north-western Himalaya. Chem. Ecol. 2021, 37, 850–865. [Google Scholar] [CrossRef]
- Pragg, C.; Mohammed, F.K. Distribution and health risk assessment of heavy metals in road dust from an industrial estate in Trinidad, West Indies. Int. J. Environ. Health Res. 2020, 30, 336–343. [Google Scholar] [CrossRef]
- Moghtaderi, M.; Ashraf, M.A.; Moghtaderi, T.; Teshnizi, S.H.; Hesamedin Nabavizadeh, S.H. Heavy metal concentration in classroom dust samples and its relationship with childhood asthma: A study from Islamic Republic of Iran. East. Mediterr. Health J. 2020, 26, 594–601. [Google Scholar] [CrossRef]
- Samai, I.; Nebbache, S.; Chalane, F.; Meghlaoui, Z.; Ramdani, H. Heavy metal contamination of Oued El Harrache surface water (Algiers-Algeria). Egypt. J. Aquat. Biol. Fish. 2023, 27, 531–550. [Google Scholar] [CrossRef]
- Ahmad, L.; Waheed, H.; Gul, N.; Sheikh, L.; Khan, A.; Iqbal, H. Geochemistry of subsurface water of Swabi district and associated health risk with heavy metal contamination. Environ. Monit. Assess. 2022, 194, 480. [Google Scholar] [CrossRef] [PubMed]
- Al-Afify, D.G.; Abdel-Satar, A.M. Heavy metal contamination of the River Nile environment, Rosetta Branch, Egypt. Water Air Soil Pollut. 2022, 233, 302. [Google Scholar] [CrossRef]
- Kara, H.; Yetis, A.D.; Temel, H. Assessment of heavy metal contamination in groundwater of Diyarbakir Oil Production Area, (Turkey) using pollution indices and chemometric analysis. Environ. Earth Sci. 2021, 80, 697. [Google Scholar] [CrossRef]
- Custodio, M.; Fow, A.; Chanamé, F.; Orellana-Mendoza, E.; Peñaloza, R.; Alvarado, J.C.; Cano, D.; Pizarro, S. Ecological Risk Due to Heavy Metal Contamination in Sediment andWater of Natural Wetlands with Tourist Influence in the Central Region of Peru. Water 2021, 13, 2256. [Google Scholar] [CrossRef]
- Dehbi, M.; Dehbi, F.; Kanjal, M.I.; Tahraoui, H.; Zamouche, M.; Amrane, A.; Assadi, A.A.; Hadadi, A.; Mouni, L. Analysis of heavy metal contamination in macroalgae from surfacewaters in Djelfa, Algeria. Water 2023, 15, 974. [Google Scholar] [CrossRef]
- Kilavi, P.K.; Kaniu, M.I.; Patel, J.P.; Usman, I.T. Quality and human health risk assessment of uranium and other heavy metals in drinking water from Kwale County, Kenya. Environ. Monit. Assess. 2021, 193, 746. [Google Scholar] [CrossRef]
- Pérez-Figueroa, C.E.; Salazar-Moreno, R.; Rodríguez, E.F.; Cruz, I.L.L.L.; Schmidt, U.; Danneh, D. Heavy metals accumulation in lettuce and cherry tomatoes cultivated in cities. Pol. J. Environ. Stud. 2023, 32, 2293–2308. [Google Scholar] [CrossRef]
- Darko, G.; Adjei, S.; Nkansah, M.A.; Borquaye, L.S.; Boakye, K.O.; Dodd, M. Accumulation and bioaccessibility of toxic metals in root tubers and soils from gold mining and farming communities in the Ashanti region of Ghana. Int. J. Environ. Health Res. 2022, 32, 426–436. [Google Scholar] [CrossRef]
- Kumar, M.; Mohapatra, S.; Karim, A.A.; Dhal, N.K. Heavy metal fractions in rhizosphere sediment vis-à-vis accumulation in Phoenix paludosa (Roxb.) mangrove plants at Dhamra Estuary of India: Assessing phytoremediation potential. Chem. Ecol. 2021, 37, 1–14. [Google Scholar] [CrossRef]
- Topal, A.I.A.; Topal, M.; Öbek, E. Assessment of heavy metal accumulations and health risk potentials in tomatoes grown in the discharge area of a municipal wastewater treatment plant. Int. J. Environ. Health Res. 2022, 32, 393–405. [Google Scholar] [CrossRef]
- Asrade, B.; Ketema, G. Determination of the selected heavy metal content and its associated health risks in selected vegetables marketed in Bahir Dar town, Northwest Ethiopia. J. Food Qual. 2023, 2023, 7370171. [Google Scholar] [CrossRef]
- Tariq, Y.; Ehsan, N.; Riaz, U.; Nasir, R.; Khan, W.A.; Iqbal, R.; Ali, S.; Mahmoud, E.A.; Ullah, I.; Elansary, H.O. Assessment of Heavy Metal(oid)s Accumulation in eggplant and soil under different irrigation systems. Water 2023, 15, 1049. [Google Scholar] [CrossRef]
- Murtaza, G.; Shehzad, M.T.; Kanwal, S.; Farooqi, Z.U.R.; Owens, G. Biomagnification of potentially toxic elements in animals consuming fodder irrigated with sewage water. Environ. Geochem. Health 2022, 44, 4523–4538. [Google Scholar] [CrossRef] [PubMed]
- Joshua, G.; Ali, Z.; Ayub, M.; Nadeem, S.I. Heavy metal contamination in wild avian species inhabiting human-modified habitats. Environ. Monit. Assess. 2021, 193, 588. [Google Scholar] [CrossRef] [PubMed]
- Köker, L.; Aydın, F.; Gaygusuz, Ö.; Akçaalan, R.; Çamur, D.; İlter, H.; Ayoğlu, F.N.; Altın, A.; Topbaş, M.; Albay, M. Heavy Metal Concentrations in Trachurus Mediterraneus and Merlangius Merlangus Captured from Marmara Sea, Turkey and Associated Health Risks. Environ. Manag. 2021, 67, 522–531. [Google Scholar] [CrossRef]
- Collin, M.S.; Venkatraman, S.K.; Vijayakumar, N.; Kanimozhi, V.; Arbaaz, S.M.; Stacey, R.G.S.; Anusha, J.; Choudhary, R.; Lvov, V.; Tovar, G.I.; et al. Bioaccumulation of lead (Pb) and its effects on human: A review. J. Hazard. Mater. Adv. 2022, 7, 100094. [Google Scholar] [CrossRef]
- Pham, V.H.T.; Kim, J.; Chang, S.; Chung, W. Bacterial biosorbents, an efficient heavy metals green clean-Up strategy: Prospects, challenges, and opportunities. Microorganisms 2022, 10, 610. [Google Scholar] [CrossRef]
- Haseeb, A.; Fozia; Ahmad, I.; Ullah, H.; Iqbal, A.; Ullah, R.; Moharram, B.A.; Kowalczyk, A. Ecotoxicological assessment of heavy metal and its biochemical effect in fishes. BioMed Res. Int. 2022, 2022, 3787838. [Google Scholar] [CrossRef]
- Banu, R.; Jahan, B.; Mondal, N.; Hossain, A. Toxicity effects of metals bioaccumulation in water and fishes of the Balu River, Bangladesh. Egypt. J. Aquat. Biol. Fish. 2023, 27, 271–294. [Google Scholar] [CrossRef]
- Guo, Z.; Gao, Y.; Yuan, X.; Yuan, M.; Huang, L.; Wang, S.; Liu, C.; Duan, C. Effects of Heavy Metals on Stomata in Plants: A Review. Int. J. Mol. Sci. 2023, 24, 9302. [Google Scholar] [CrossRef]
- Khan, Z.; Tariq, E.; Hadi, S. Heavy metal toxicological status of wheat samples from district swabi, pakistan. J. Anim. Plant Sci. 2022, 32, 1317–1322. [Google Scholar] [CrossRef]
- Riyazuddin, R.; Nisha, N.; Ejaz, B.; Khan, M.I.R.; Kumar, M.; Ramteke, P.W.; Gupta, R. A comprehensive review on the heavy metal toxicity and sequestration in plants. Biomolecules 2022, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Wang, H.X.; Wang, Y.; Hu, X.Q.; Wen, X.L. Effects of Cd2+ and Pb2+ on growth and photosynthesis of two freshwater algae species. Pol. J. Environ. Stud. 2022, 31, 2059–2068. [Google Scholar] [CrossRef]
- Proshad, R.; Islamc, S.; Tusher, T.R.; Zhanga, D.; Khadka, S.; Jianing Gao, J.; Kundu, S. Appraisal of heavy metal toxicity in surface water with human health risk by a novel approach: A study on an urban river in vicinity to industrial areas of Bangladesh. Toxin Rev. 2021, 40, 803–819. [Google Scholar] [CrossRef]
- Amponsah, L.O.; Dodd, M.; Darko, G. Gastric bioaccessibility and human health risks associated with soil metal exposure via ingestion at an E-waste recycling site in Kumasi, Ghana. Environ. Geochem. Health 2022, 44, 497–509. [Google Scholar] [CrossRef]
- Mandal, R.; Kaur, S.; Gupta, V.K.; Joshi, A. Heavy metals controlling cardiovascular diseases risk factors in myocardial infarction patients in critically environmentally heavy metal-polluted steel industrial town Mandi-Gobindgarh (India). Environ. Geochem. Health 2022, 44, 3215–3238. [Google Scholar] [CrossRef]
- Hendryx, M.; Luo, J.; Chojenta, C.; Byles, J.E. Exposure to heavy metals from point pollution sources and risk of incident type 2 diabetes among women: A prospective cohort analysis. Int. J. Environ. Health Res. 2021, 31, 453–464. [Google Scholar] [CrossRef]
- Nowicka, B. Heavy metal–induced stress in eukaryotic algae—Mechanisms of heavy metal toxicity and tolerance with particular emphasis on oxidative stress in exposed cells and the role of antioxidant response. Environ. Sci. Pollut. Res. 2022, 29, 16860–16911. [Google Scholar] [CrossRef]
- Fu, Z.; Xi, S. The effects of heavy metals on human metabolism. Toxicol. Mech. Methods 2020, 30, 167–176. [Google Scholar] [CrossRef]
- Amadi, C.N.; Igweze, Z.N.; Orisakwe, O.E. Heavy metals in miscarriages and stillbirths in developing nations. Middle East. Fertil. Soc. J. 2017, 22, 91–100. [Google Scholar] [CrossRef]
- Guzzi, G.; La Porta, C.A. Molecular mechanisms triggered by mercury. Toxicology 2008, 244, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Davidson, P.W.; Myers, G.J.; Weiss, B. Mercury exposure and child development outcomes. Pediatrics 2004, 113 (Suppl. S3), 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.R.; Prozialeck, W.C. Cadmium, diabetes and chronic kidney disease. Toxicol. Appl. Pharmacol. 2009, 238, 289–293. [Google Scholar] [CrossRef]
- Shahid, M.; Pourrut, B.; Dumat, C.; Nadeem, M.; Aslam, M.; Pinelli, E. Heavy-metal induced reactive oxygen species: Phytotoxicity and physicochemical changes in plants. In Reviews of Environmental Contamination and Toxicology; Whitacre, D., Ed.; Springer: Cham, Switzerland, 2014; Volume 232, pp. 1–44. [Google Scholar]
- Berni, R.; Luyckx, M.; Xu, S.; Legay, K.; Sergeant, J.; Hausman, S.; Lutts, G.; Guerriero, G. Reactive oxygen species and heavy metal stress in plants: Impact on the cell wall and secondary metabolism. Environ. Exp. Bot. 2019, 161, 98–106. [Google Scholar] [CrossRef]
- Kim, J.J.; Kim, Y.S.; Kumar, V. Heavy metal toxicity: An update of chelating therapeutic strategies. J. Trace Elem. Med. Biol. 2019, 54, 226–231. [Google Scholar] [CrossRef]
- Li, W.; Zu, B.; Yang, Q.W.; Huang, Y.Q.; Li, J.W. Adsorption of lead and cadmium by microplastics and their desorption behavior as vectors in the gastrointestinal environment. J. Environ. Chem. Eng. 2022, 10, 107379. [Google Scholar] [CrossRef]
- Pan, H.J.; Lakshmipriya, T.; Gopinath, S.C.B.; Anbu, P. High-Affinity detection of metal-mediated nephrotoxicity by aptamer nanomaterial complementation. Curr. Nanosci. 2019, 15, 549–556. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Z.; Zhang, L.; Meng, R.N.; Gao, J.; Jin, M.; Li, M.; Wang, X.P. Effect of metal ions on Alzheimer’s disease. Brain Behav. 2022, 12, e2527. [Google Scholar] [CrossRef]
- Boffetta, P. Carcinogenicity of trace elements with reference to evaluations made by the International Agency for Research on Cancer. Scand. J. Work. Environ. Health 1993, 19 (Suppl. S1), 67–70. [Google Scholar]
- Goyer, R.A.; Liu, J.; Waalkes, M.P. Cadmium and cancer of prostate and testis. Biometals 2004, 17, 555–558. [Google Scholar] [CrossRef]
- Persico, M.; Perrotta, S.; Persico, E.; Terracciano, L.; Folgori, A.; Ruggeri, L.; Masarone, M. Environmental exposure to cadmium and risk of cancer: A prospective population based study. Lancet Oncol. 2006, 7, 119–126. [Google Scholar]
- Pieper, K.J.; Martin, R.; Tang, M.; Walters, L.; Parks, J.; Roy, S.; Devine, C.; Marc, A.; Edwards, M.A. Evaluating water lead levels during the Flint water crisis. Environ. Sci. Technol. 2018, 52, 8124–8132. [Google Scholar] [CrossRef] [PubMed]
- Pieper, K.J.; Tang, M.; Edwards, M.A. Flint water crisis caused by interrupted corrosion control: Investigating “Ground Zero” Home. Environ. Sci. Technol. 2017, 51, 2007–2014. [Google Scholar] [CrossRef] [PubMed]
- Hanna-Attisha, M.; LaChance, J.; Sadler, R.C.; Schnepp, A.C. Elevated blood lead levels in children associated with the Flint drinking water crisis: A spatial analysis of risk and public health response. Am. J. Public Health 2016, 106, 283–290. [Google Scholar] [CrossRef]
- Sadler, R.C.; LaChance, J.; Hanna-Attisha, M. Social and built environmental correlates of predicted blood lead levels in the Flint water crisis. Am. J. Public Health 2017, 107, 763–769. [Google Scholar] [CrossRef]
- Kruger, D.J.; Cupal, S.; Franzen, S.P.; Kodjebacheva, G.; Bailey, E.S.; Key, K.D.; Kaufman, M.M. Toxic trauma: Householdwater quality experiences predict posttraumatic stress disorder symptoms during the Flint, Michigan, water crisis. J. Community Psychol. 2017, 45, 957–962. [Google Scholar] [CrossRef]
- Wang, R.; Chen, X.; Li, X. Something in the pipe: The Flint water crisis and health at birth. J. Popul. Econ. 2022, 35, 1723–1749. [Google Scholar] [CrossRef]
- Kruger, D.J.; Cupal, S.; Kodjebacheva, G.D.; Fockler, T.V. Perceived water quality and reported health among adults during the Flint, MI water crisis. Californian J. Health Promot. 2017, 15, 56–61. [Google Scholar] [CrossRef]
- Ezell, J.M.; Chase, E.C. A Population-based assessment of physical symptoms and mental health outcomes among adults following the Flint water crisis. J. Urban Health 2021, 98, 642–653. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA; American Water Works Association: Denver, CO, USA; Water Environment Federation: Alexandria, VA, USA, 2002; Volume 2, pp. 1–541. [Google Scholar]
- Ramdani, S.; Amar, A.; Belhsaien, K.; El Hajjaji, S.; Ghalem, S.; Zouahri, A. Assessment of heavy metal pollution and ecological risk of roadside soils in Tlemcen (Algeria) using flame-atomic absorption spectrometry. Anal. Lett. 2018, 51, 2468–2487. [Google Scholar] [CrossRef]
- Otero-Romaní, J.; Moreda-Piñ, A.; Bermejo-Barrera, P. Evaluation of commercial C18 cartridges for trace elements solid phase extraction from seawater followed by inductively coupled plasma-optical emission spectrometry determination. Anal. Chim. Acta 2005, 536, 213–218. [Google Scholar] [CrossRef]
- Yan, N.; Zhu, Z.; Jin, L.; Guo, W.; Gan, Y.; Hu, S. Quantitative characterization of gold nanoparticles by coupling thin layer chromatography with laser ablation inductively coupled plasma mass spectrometry. Anal. Chem. 2015, 87, 6079–6087. [Google Scholar] [CrossRef] [PubMed]
- Hamida, S.; Ouabdesslam, L.; Ladjel, A.; Escudero, M.; Anzano, J. Determination of cadmium, copper, lead, and zinc in pilchard sardines from the Bay of Boumerdés by atomic absorption spectrometry. Anal. Lett. 2018, 51, 2501–2508. [Google Scholar] [CrossRef]
- Madden, J.T.; Fitzgerald, N. Investigation of ultraviolet photolysis vapor generation with in-atomizer trapping graphite furnace atomic absorption spectrometry for the determination of mercury. Spectrochim. Acta B 2009, 64, 925–927. [Google Scholar] [CrossRef]
- Kristian, K.E.; Friedbauer, S.; Kabashi, D.; Ferencz, K.M.; Kelly, O. A simplified digestion protocol for the analysis of Hg in fish by cold vapor atomic absorption spectroscopy. J. Chem. Educ. 2015, 92, 698–702. [Google Scholar] [CrossRef]
- Jaswal, B.B.S.; Rai, P.K.; Singh, T.; Zorba, V.; Singh, V.K. Detection and quantification of heavy metal elements in gallstones using X-ray fluorescence spectrometry. X-ray Spectrom. 2019, 48, 178–187. [Google Scholar] [CrossRef]
- Mohamed, R.; Zainudin, B.H.; Yaakob, A.S. Method validation and determination of heavy metals in cocoa beans and cocoa products by microwave assisted digestion technique with inductively coupled plasmamass spectrometry. Food Chem. 2020, 303, 125392. [Google Scholar] [CrossRef] [PubMed]
- Dadfarnia, S.; Assadollahi, T.; Shabani, A.M.H. Speciation and determination of thallium by on-line microcolumn separation/preconcentration by flow injection-flame atomic absorption spectrometry using immobilized oxine as sorbent. J. Hazard. Mater. 2007, 148, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Zachariadis, G.A.; Themelis, D.G.; Kosseoglou, D.J.; Stratis, J.A. Flame AAS and UV-VIS determination of cobalt, nickel and palladium using the synergetic effect of 2-benzoylpyridine-2-pyridylhydrazone and thiocyanate ions. Talanta 1998, 47, 161–167. [Google Scholar] [CrossRef]
- Thines, L.; Iserentant, A.; Morsomme, P. Determination of the Cellular Ion Concentration in Saccharomyces cerevisiae Using ICP-AES. Bio-Protocol 2020, 10, 3727. [Google Scholar] [CrossRef]
- Chunqiang, L.U.; Sun, D.; Sun, K.; Liu, J.; Luo, C. Determination of the three harmful heavy mental ions in plant fiber molded products by ICP-MS. Food Ind. 2019, 40, 299–301. [Google Scholar]
- Hołyńska, B.; Ostachowicz, B.; Wȩgrzynek, D. Simple method of determination of copper, mercury and lead in potable water with preliminary pre-concentration by total reflection X-ray fluorescence spectrometry. Spectrochim. Acta B At. Spectrosc. 1996, 51, 769–773. [Google Scholar] [CrossRef]
- Hu, T.; Lai, Q.; Fan, W.; Zhang, Y.; Liu, Z. Advances in Portable Heavy Metal Ion Sensors. Sensors 2023, 23, 4125. [Google Scholar] [CrossRef] [PubMed]
- Ullah, N.; Mansha, M.; Khan, I.; Qurashi, A. Nanomaterial-based optical, chemical sensors for detecting heavy metals in water: Recent advances and challenges. TrAC Trends Anal. Chem. 2018, 100, 155–166. [Google Scholar] [CrossRef]
- GadelHak, Y.; Hafez, S.H.M.; Mohamed, H.F.M.; Abdel-Hady, E.E.; Mahmoud, R. Nanomaterials-modified disposable electrodes and portable electrochemical systems for heavy metals detection in wastewater streams: A review. Microchem. J. 2023, 193, 109043. [Google Scholar] [CrossRef]
- Ding, Q.; Li, C.; Wang, H.; Xu, C.; Kuang, H. Electrochemical detection of heavy metal ions in water. Chem. Commun. 2021, 57, 7215–7231. [Google Scholar] [CrossRef]
- Nayan Kumar, H.N.; Nagaraju, D.H.; Yhobu, Z.; Shivakumar, P.; Manjunatha Kumara, K.S.; Budagumpi, S.; Praveen, B.M. Recent advances in on-site monitoring of heavy metal ions in the environment. Microchem. J. 2022, 182, 107894. [Google Scholar] [CrossRef]
- Garcia-Miranda Ferrari, A.; Carrington, P.; Rowley-Neale, S.J.; Banks, C.E. Recent advances in portable heavy metal electrochemical sensing platforms. Environ. Sci. Water Res. Technol. 2020, 6, 2676–2690. [Google Scholar] [CrossRef]
- Li, Z.; Xu, D.; Zhang, D.; Yamaguchi, Y. A portable instrument for on-site detection of heavy metal ions in water. Anal. Bioanal. Chem. 2021, 413, 3471–3477. [Google Scholar] [CrossRef]
- Thakur, A.; Kumar, A. Recent advances on rapid detection and remediation of environmental pollutants utilizing nanomaterials-based (bio)sensors. Sci. Total Environ. 2022, 834, 155219. [Google Scholar] [CrossRef]
- Mohamad Nor, N.; Ramli, N.H.; Poobalan, H.; Qi Tan, K.; Abdul Razak, K. Recent Advancement in Disposable Electrode Modified with Nanomaterials for Electrochemical Heavy Metal Sensors. Crit. Rev. Anal. Chem. 2023, 53, 253–288. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Xiong, Y.; Ge, Y.; Wen, Y.; Zeng, X.; Zhang, J.; Wang, P.; Wang, Z.; Chen, S. Magnetic Fe3O4 nanoparticles decorated phosphorus-doped biochar-attapulgite/bismuth film electrode for smartphone-operated wireless portable sensing of ultra-trace multiple heavy metal ions. Microchim. Acta 2023, 190, 94. [Google Scholar] [CrossRef] [PubMed]
- Butmee, P.; Mala, J.; Damphathik, C.; Kunpatee, K.; Tumcharern, G.; Kerr, M.; Mehmeti, E.; Raber, G.; Kalcher, K.; Samphao, A. A portable selective electrochemical sensor amplified with Fe3O4@Au-cysteamine-thymine acetic acid as conductive mediator for determination of mercuric ion. Talanta 2021, 221, 121669. [Google Scholar] [CrossRef] [PubMed]
- Naseri, M.; Mohammadniaei, M.; Ghosh, K.; Sarkar, S.; Sankar, R.; Mukherjee, S.; Pal, S.; Ansari Dezfouli, E.; Halder, A.; Qiao, J.; et al. A Robust Electrochemical Sensor Based on Butterfly-shaped Silver Nanostructure for Concurrent Quantification of Heavy Metals in Water Samples. Electroanalysis 2023, 35, e202200114. [Google Scholar] [CrossRef]
- Saenchoopa, A.; Klangphukhiew, S.; Somsub, R.; Talodthaisong, C.; Patramanon, R.; Daduang, J.; Daduang, S.; Kulchat, S. A Disposable Electrochemical Biosensor Based on Screen-Printed Carbon Electrodes Modified with Silver Nanowires/HPMC/Chitosan/Urease for the Detection of Mercury (II) in Water. Biosensors 2021, 11, 351. [Google Scholar] [CrossRef]
- Hajzus, J.R.; Shriver-Lake, L.C.; Dean, S.N.; Erickson, J.S.; Zabetakis, D.; Golden, J.; Pennachio, D.J.; Myers-Ward, R.L.; Trammell, S.A. Modifications of Epitaxial Graphene on SiC for the Electrochemical Detection and Identification of Heavy Metal Salts in Seawater. Sensors 2022, 22, 5367. [Google Scholar] [CrossRef]
- Bao, Q.; Li, G.; Yang, Z.; Pan, P.; Liu, J.; Li, R.; Wei, J.; Hu, W.; Cheng, W.; Lin, L. In situ detection of heavy metal ions in sewage with screen-printed electrode-based portable electrochemical sensors. Analyst 2021, 146, 5610–5618. [Google Scholar] [CrossRef]
- Tan, B.; Yuan, R.; Xie, X.; Qi, Y.; Qi, Z.; Wang, X. High performance Hetero-Shelled hollow structure Metal-Organic framework hybrid material for the efficient electrochemical determination of lead ions. Microchem. J. 2023, 193, 109147. [Google Scholar] [CrossRef]
- Qi, Y.; Chen, X.; Liu, S.; Yang, P.; Zhang, S.; Hou, C.; Huo, D. Electrochemical sensor for Cd2+ detection based on carbon fiber paper sequentially modified with CoMOF, AuNPs, and glutathione. J. Electrochem. Soc. 2021, 168, 067526. [Google Scholar] [CrossRef]
- Wang, C.; Pei, L.; Chen, R.; Zhu, Y.; Su, J. A portable screen-printing electrode modified by COFDATA-TP with abundant carboxyl and secondary amine groups for simultaneous detection of Hg2+, Cu2+, Pb2+, and Cd2+. Ionics 2022, 28, 4025–4033. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, G.; Yang, W.; Dai, X.; Huang, Y.; Ni, J.; Wang, Q. Portable anti-fouling electrochemical sensor for soil heavy metal ions detection based on the screen-printed carbon electrode modified with silica isoporous membrane. J. Electroanal. Chem. 2023, 930, 117141. [Google Scholar] [CrossRef]
- Rechotnek, F.; Follmann, H.D.M.; Silva, R. Mesoporous silica decorated with L-cysteine as active hybrid materials for electrochemical sensing of heavy metals. J. Environ. Chem. Eng. 2021, 9, 106492. [Google Scholar] [CrossRef]
- Costa, M.; Di Masi, S.; Garcia-Cruz, A.; Piletsky, S.A.; Malitesta, C. Disposable electrochemical sensor based on ion imprinted polymeric receptor for Cd(II) ion monitoring in waters. Sens. Actuators B 2023, 383, 133559. [Google Scholar] [CrossRef]
- Li, H.; Zhao, J.; Zhao, S.; Cui, G. Simultaneous determination of trace Pb(II), Cd(II), and Zn(II) using an integrated three-electrode modified with bismuth film. Microchem. J. 2021, 168, 106390. [Google Scholar] [CrossRef]
- Bu, L.; Xie, Q.; Ming, H. Simultaneous sensitive analysis of Cd(II), Pb(II) and As(III) using a dual-channel anodic stripping voltammetry approach. New J. Chem. 2020, 44, 5739–5745. [Google Scholar] [CrossRef]
- Heavy Metals Screen with Demographics, Blood. Available online: https://www.mayocliniclabs.com/test-catalog/overview/39183 (accessed on 26 September 2023).
- Heavy Metal/Creatinine Ratio, with Reflex, Random, Urine. Available online: https://www.mayocliniclabs.com/test-catalog/overview/608899 (accessed on 6 September 2023).
- Faheem, A.; Cinti, S. Non-invasive electrochemistry-driven metals tracing in human biofluids. Biosens. Bioelectron. 2022, 200, 113904. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.R.; Raymundo-Pereira, P.A.; Campos, A.M.; Wilson, D.; Otoni, C.G.; Barud, H.S.; Costa, C.A.R.; Domeneguetti, R.R.; Balogh, D.T.; Ribeiro, S.J.L.; et al. Microbial nanocellulose adherent to human skin used in electrochemical sensors to detect metal ions and biomarkers in sweat. Talanta 2020, 218, 121153. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Zhao, W.; Zhang, Q.; Zhang, K.; Liang, C.; Wang, D.; Liu, X.; Zhan, X. A portable microfluidic electrochemical sensing platform for rapid detection of hazardous metal Pb2+ based on thermocapillary convection using 3D Ag-rGO-f-Ni(OH)2/NF as a signal amplifying element. J. Hazard. Mater. 2023, 448, 130923. [Google Scholar] [CrossRef]
- Wang, W.; Ding, S.; Wang, Z.; Lv, Q.; Zhang, Q. Electrochemical paper-based microfluidic device for on-line isolation of proteins and direct detection of lead in urine. Biosens. Bioelectron. 2021, 187, 113310. [Google Scholar] [CrossRef] [PubMed]
- Raucci, A.; Miglione, A.; Spinelli, M.; Amoresano, A.; Cinti, S. A hybrid screen-printed strip for enhanced electroanalysis towards lead and cadmium in multi-matrices. J. Electrochem. Soc. 2022, 169, 037516. [Google Scholar] [CrossRef]
- Gazica, K.; FitzGerald, E.; Dangel, G.; Haynes, E.N.; Yadav, J.; Alvarez, N.T. Towards on-site detection of cadmium in human urine. J. Electroanal. Chem. 2020, 859, 113808. [Google Scholar] [CrossRef]
- Baile, P.; Vidal, L.; Canals, A. Magnetic dispersive solid-phase extraction using ZSM-5 zeolite/Fe2O3 composite coupled with screen-printed electrodes based electrochemical detector for determination of cadmium in urine samples. Talanta 2020, 220, 121394. [Google Scholar] [CrossRef] [PubMed]
- Mohan, J.M.; Dudala, S.; Amreen, K.; Javed, A.; Dubey, S.K.; Goel, S. Microfluidic Device Integrated with PDMS Microchannel and unmodified ITO glass Electrodes for Highly Sensitive, Specific and Point-of Care Detection of Copper and Mercury. IEEE Trans. NanoBiosci. 2023, 22, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Vieira, D.; Allard, J.; Taylor, K.; Harvey, E.J.; Merle, G. Zincon-Modified CNTs Electrochemical Tool for Salivary and Urinary Zinc Detection. Nanomaterials 2022, 12, 4431. [Google Scholar] [CrossRef]
- Shan, Y.; Lu, Y.-N.; Yi, W.; Wang, B.; Li, J.; Guo, J.; Li, W.; Yin, Y.; Wang, S.; Liu, F. On-site food safety detection: Opportunities, advancements, and prospects. Biosens. Bioelectron. X 2023, 14, 100350. [Google Scholar] [CrossRef]
- Wang, B.; Huang, D.; Weng, Z. Recent Advances in Polymer-Based Biosensors for Food Safety Detection. Polymers 2023, 15, 3253. [Google Scholar] [CrossRef]
- Kuswandi, B.; Hidayat, M.A.; Noviana, E. Paper-Based Electrochemical Biosensors for Food Safety Analysis. Biosensors 2022, 12, 1088. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bhattacharyya, S.; Ghosh, K.; Pal, S.; Halder, A.; Naseri, M.; Mohammadniaei, M.; Sarkar, S.; Ghosh, A.; Sun, Y.; et al. Sensory development for heavy metal detection: A review on translation from conventional analysis to field-portable sensor. Trends Food Sci. Technol. 2021, 109, 674–689. [Google Scholar] [CrossRef]
- Pang, Y.-H.; Yang, Q.-Y.; Jiang, R.; Wang, Y.-Y.; Shen, X.F. A stack-up electrochemical device based on metal-organic framework modified carbon paper for ultra-trace lead and cadmium ions detection. Food Chem. 2023, 398, 133822. [Google Scholar] [CrossRef]
- Jiang, D.; Sheng, K.; Gui, G.; Jiang, H.; Liu, X.; Wang, L. A novel smartphone-based electrochemical cell sensor for evaluating the toxicity of heavy metal ions Cd2+, Hg2+, and Pb2+ in rice. Anal. Bioanal. Chem. 2021, 413, 4277–4287. [Google Scholar] [CrossRef]
- Palisoc, S.T.; Chua, R.V.M.; Natividad, M.T. Highly sensitive determination of heavy metals in upland and lowland rice using AgNP/BiNP/MWCNT/nafion modified glassy carbon electrode via anodic stripping voltammetry. Mater. Res. Express 2020, 7, 015081. [Google Scholar] [CrossRef]
- Bansod, B.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens. Bioelectron. 2017, 94, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, T.; Shafi, S.; Ali, J.; Sher, F.; Rizwan, K.; Khan, S. Recent advances in chemically and biologically synthesized nanostructures for colorimetric detection of heavy metal. J. King Saud Univ. Sci. 2022, 34, 101745. [Google Scholar] [CrossRef]
- Xu, X.; Yang, S.; Wang, Y.; Qian, K. Nanomaterial-based sensors and strategies for heavy metal ion detection. Green Anal. Chem. 2022, 2, 100020. [Google Scholar] [CrossRef]
- Wu, Y.; Feng, J.; Hu, G.; Zhang, E.; Yu, H.-H. Colorimetric sensors for chemical and biological sensing applications. Sensors 2023, 23, 2749. [Google Scholar] [CrossRef]
- Ghasemi, Z.; Mohammadi, A. Sensitive and selective colorimetric detection of Cu (II) in water samples by thiazolylazopyrimidine-functionalized TiO2 nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 239, 118554. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, Y.; Pang, J.; Sun, J.; Yang, F.; Li, H.; Liu, Y. Iodide-assisted silver nanoplates for colorimetric determination of chromium(III) and copper(II) via an aggregation/fusion/oxidation etching strategy. Microchim. Acta 2020, 187, 19. [Google Scholar] [CrossRef]
- Aygun, A.; Sahin, G.; Elhouda Tiri, R.N.; Tekeli, Y.; Sen, F. Colorimetric sensor based on biogenic nanomaterials for high sensitive detection of hydrogen peroxide and multi-metals. Chemosphere 2023, 339, 139702. [Google Scholar] [CrossRef]
- Li, X.; Zhao, C.-X.; Lin, L. Plasma-based instant synthesis of functionalized gold nanoparticles for colorimetric detection of lead ions. Chem. Eng. Sci. 2022, 260, 117849. [Google Scholar] [CrossRef]
- Tian, H.; Liu, J.; Guo, J.; Cao, L.; He, J. L-Cysteine functionalized graphene oxide nanoarchitectonics: A metal-free Hg2+ nanosensor with peroxidase-like activity boosted by competitive adsorption. Talanta 2022, 242, 123320. [Google Scholar] [CrossRef]
- Faghiri, F.; Ghorbani, F. Synthesis of graphene oxide nanosheets from sugar beet bagasse and its application for colorimetric and naked eye detection of trace Hg2+ in environmental water samples. Microchem. J. 2020, 152, 104332. [Google Scholar] [CrossRef]
- Qi, Y.; Ma, J.; Chen, X.; Xiu, F.-R.; Chen, Y.; Lu, Y. Practical aptamer-based assay of heavy metal mercury ion in contaminated environmental samples: Convenience and sensitivity. Anal. Bioanal. Chem. 2020, 412, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Kheibarian, Z.; Soleimani, E.; Mardani, H.R. Green synthesis of Cu@Ag core-shell nanoparticles as efficient colorimetric sensing for Hg(II) ion. Appl. Phys. A 2022, 128, 466. [Google Scholar] [CrossRef]
- Chadha, R.; Das, A.; Debnath, A.K.; Kapoor, S.; Maiti, N. 2-thiazoline-2-thiol functionalized gold nanoparticles for detection of heavy metals, Hg(II) and Pb(II) and probing their competitive surface reactivity: A colorimetric, surface enhanced Raman scattering (SERS) and X-ray photoelectron spectroscopy (XPS) study. Colloids Surf. A Physicochem. Eng. 2021, 615, 126279. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Zhou, P.; Tao, H.; Wang, X.; Wu, Y. Oligonucleotide-induced regulation of oxidase-mimicking activity of octahedral Mn3O4 nanoparticles for colorimetric detection of heavy metals. Microchim. Acta 2020, 187, 99. [Google Scholar] [CrossRef]
- Mohammadzadeh, S.E.; Faghiri, F.; Ghorbani, F. Green synthesis of phenolic capping Ag NPs by green walnut husk extract and its application for colorimetric detection of Cd2+ and Ni2+ ions in environmental samples. Microchem. J. 2022, 179, 107475. [Google Scholar] [CrossRef]
- Ullah, L.; Ali, S.; Ullah, A.; Khan, M.; Imran, M.; Shah, R.; Jun, L. Synthesis of acyclovir stabilized silver nanoparticles for selective recognition of Hg2+ in different media. Int. J. Environ. Sci. Technol. 2022, 19, 11279–11290. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, C.; Chu, S.; Li, S.; Wang, F.; Si, Y.; Mao, G.; Wu, C.; Wang, H. Covalent organic framework- loaded silver nanoparticles as a robust mimetic oxidase for highly sensitive and selective colorimetric detection of mercury in blood. J. Mater. Chem. B 2022, 10, 10075. [Google Scholar] [CrossRef]
- Shalvi, N.K.; Kanak, L.V.; Vinod, K.J.; Suman, N. Integrated device for colorimetric determination of arsenite using polyethylene glycol capped gold nanoparticles—Lab-on-chip. Toxicol. Environ. Health Sci. 2021, 13, 351–362. [Google Scholar] [CrossRef]
- Zhang, D.; Chu, S.; Wang, L.; Zhan, X.; Zhou, P.; Zhang, D. Dual-mode colorimetric determination of as (III) based on negatively-charged aptamer-mediated aggregation of positively-charged AuNPs. Anal. Chim. Acta 2022, 1221, 340111. [Google Scholar] [CrossRef]
- Sonia; Seth, R. L-Cysteine Functionalized Gold Nanoparticles as a Colorimetric Sensor for Ultrasensitive Detection of Toxic Metal Ion Cadmium. Mater. Today Proc. 2020, 24, 2375–2382. [Google Scholar] [CrossRef]
- Vonnie, J.M.; Rovina, K.; Erna, K.H.; Manthial, S.; Huda, N.; Wahab, R.A. Development of a colorimetric sensor based on tapioca starch and gold nanoparticles for detection of cadmium particles in fish products. J. Food Nutr. Res. 2022, 61, 315–322. [Google Scholar]
- Sharma, S.; Jaiswal, A.; Uttam, K.N. Colorimetric and surface enhanced raman scattering (SERS) detection of metal ions in aqueous medium using sensitive, robust and novel pectin functionalized silver nanoparticles. Anal. Lett. 2020, 53, 2355–2378. [Google Scholar] [CrossRef]
- Occupational Health and Safety Administration. Available online: https://www.osha.gov>cadmium (accessed on 28 September 2023).
- Yan, M.; Niu, C.; Li, X.; Wang, F.; Jiang, S.; Li, K.; Yao, Z. Heavy metal levels in milk and dairy products and health assessment: A systematic review of studies in China. Sci. Total Environ. 2022, 851 Pt 1, 158161. [Google Scholar] [CrossRef] [PubMed]




| Heavy Metal Target | Nanosensor Material | Observed/Detected Color Change (Absorbance Wavelength of Interest) | Sample Type Examined | LOD | Linear Range | Ref. |
|---|---|---|---|---|---|---|
| Environmental Samples | ||||||
| Cu2+ | Thiazolylazopyrimidine-functionalized TiO2 nanosensor (TiO2-TAP) | Yellow to red (A536) | Water | 2.51 nM | 0.01–12.5 µM | [130] |
| Cr3+ and Cu2+ | Multi-functional iodide-assisted silver nanoplates | Deep yellow to purple (A390/A520) for Cr3+; Deep yellow to colorless (A390) for Cu2+ | Environmental water samples | 8.0 nM for Cr3+; 0.27 µM for Cu2+ | 25–400 nM for Cr3+; 0.3–10 µM for Cu2+ | [131] |
| Fe3+, Cu2+, and Cr6+ | Ag@AgCl NPs | Dark brown to light brown for Fe3+; Dark brown to white for Cu2+; Dark brown to orange for Cr6+ (A400–500) | Environmental water samples | 1.69 ppb for Fe3+; 3.18 ppb for Cu2+; 5.05 ppb for Cr6+ | 0–100 ppb | [132] |
| Pb2+ | G-AuNPs | Claret-red to gray (A530) | Environmental water samples | 1.07 µM | 10–80 µM | [133] |
| Hg2+ | L-Cysteine functionalized graphene oxide nanoarchitectonics CGO | Darker blue color of TMB oxidation products (A652) | Water | 7.6 µgL−1 | 0–200 µgL−1 | [134] |
| Hg2+ | Graphene oxide stabilized AgNPs | Yellow to colorless (A400) | Environmental water samples | 0.64 nM | 10–100 µM | [135] |
| Hg2+ | Aptamer-modified cationic AuNPs | Blue to red (A560/A700) | Environmental water samples | 4.9 × 10−11 M | 8.2 × 10−10 M~6.2 × 10−8 M | [136] |
| Hg2+ | Cu@Ag NPs, stabilized with Citrus paradisi peel | Yellow to pink (A492/A411) | Aqueous solutions | 5 × 10−6 M | Not reported | [137] |
| Hg2+ and Pb2+ | 2-thiazoline-2-thiol functionalized AuNPs | Bright red to purple for (Hg2+); bright red to blue for (Pb2+) (A521) | Water samples | ~100 ppb | 0.1–10 µM | [138] |
| Hg2+ and Cd2+ | ssDNA (Hg) functionalized Mn3O4NPs | Light green-yellow (A450) | Water samples | 3.8 µgL−1 for Hg2+ and 2.4 µgL−1 for Cd2+ | Not reported | [139] |
| Cd2+ and Ni2+ | PC-Ag NPs | Brownish-yellow to pale yellow (A445) | Environmental water samples | 0.2 nM | 0.05–100 µM | [140] |
| Biological Samples | ||||||
| Hg2+ | Acyclovir stabilized, silver nanoparticles AC-AgNPs | Yellow to greyish (A404) | Human blood plasma | 0.00035 mM | Not reported | [141] |
| Hg2+ | Silver nanoparticles on covalent organic frameworks COF-Ag nanozymes | Dark blue color of TMB oxidation products (A652) | Human blood | 3.7 nM | 0.050–10 µM | [142] |
| As3+ | Polyethylene glycol-capped gold nanoparticles (PEG-AuNPs) | Wine red to blue (A612/A521) | Human tissues (viscera) | 2.9 ppm | 0.1–10 ppm | [143] |
| As3+ | As3+ aptamer functionalized positively charged gold nanoparticle. As3+ -apt- +AuNPs | Blue to red (A680/A526) | Urine | 0.41 ppb | 2–40 ppb | [144] |
| Consumables | ||||||
| Cd2+ | L-Cysteine modified gold nanoparticles AuNPs | Red to blue (A520) | Milk | Not reported | Not reported | [145] |
| Cd2+ | Film of Tapioca starch and gold nanoparticles Ts-AuNPs | Red-purplish to grey (A620) | Fish | 13.1 mmol·L−1 | 6–12 mmol·L−1 | [146] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fakayode, S.O.; Walgama, C.; Fernand Narcisse, V.E.; Grant, C. Electrochemical and Colorimetric Nanosensors for Detection of Heavy Metal Ions: A Review. Sensors 2023, 23, 9080. https://doi.org/10.3390/s23229080
Fakayode SO, Walgama C, Fernand Narcisse VE, Grant C. Electrochemical and Colorimetric Nanosensors for Detection of Heavy Metal Ions: A Review. Sensors. 2023; 23(22):9080. https://doi.org/10.3390/s23229080
Chicago/Turabian StyleFakayode, Sayo O., Charuksha Walgama, Vivian E. Fernand Narcisse, and Cidya Grant. 2023. "Electrochemical and Colorimetric Nanosensors for Detection of Heavy Metal Ions: A Review" Sensors 23, no. 22: 9080. https://doi.org/10.3390/s23229080