Determinants of Anemia among School-Aged Children in Mexico, the United States and Colombia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Sample
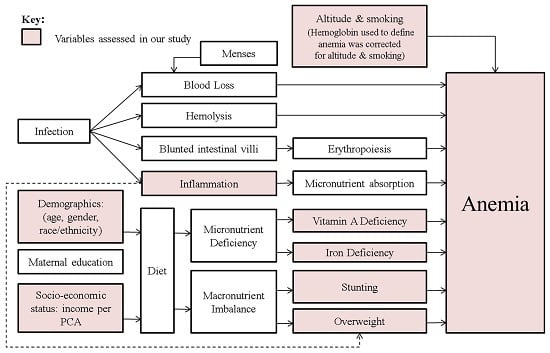
2.2. Assessment of Nutrition and Health Status
2.3. Data Management and Statistical Methods
2.4. Multivariable Modeling Approach
2.5. Ethics Statement
3. Results
3.1. Demographic and Health Characteristics
3.2. Characteristics Associated with Anemia
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| SAC | School-Aged Children |
| ID | Iron Deficiency |
| OR | Odds Ratio |
| CI | Confidence Interval |
| YLD | Years Lived with Disability |
| IDA | Iron Deficiency Anemia |
| PSC | Pre-School Children |
| CDC | Centers for Disease Control and Prevention |
| WRA | women of reproductive age |
| Hb | Hemoglobin |
| CRP | C-reactive protein |
| AGP | α-1-acid glycoprotein |
| sTFR | soluble transferrin receptor |
| ENSANUT | Mexican National Health and Nutrition Survey 2006 |
| NHANES | The National Health and Nutrition Examination Surveys |
| ENSIN | Encuesta Nacional de la Situaci ón Nutricional |
| WHO | World Health Organization |
| SES | socio-economic status |
| PCA | Principal component analysis |
| PIR | poverty income ratio |
| SE | standard error |
| IRB | institutional review board |
References
- Balarajan, Y.; Ramakrishnan, U.; Ozaltin, E.; Shankar, A.H.; Subramanian, S.V. Anaemia in low-income and middle-income countries. Lancet 2011, 378, 2123–2135. [Google Scholar] [CrossRef]
- McLean, E.; Cogswell, M.; Egli, I.; Wojdyla, D.; de Benoist, B. Worldwide prevalence of anaemia, who vitamin and mineral nutrition information system, 1993–2005. Public Health Nutr. 2009, 12, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.P.; Chen-Edinboro, L.P.; Caulfield, L.E.; Murray-Kolb, L.E. The impact of anemia on child mortality: An updated review. Nutrients 2014, 6, 5915–5932. [Google Scholar] [CrossRef] [PubMed]
- Kyu, H.H.; Pinho, C.; Wagner, J.A.; Brown, J.C.; Bertozzi-Villa, A.; Charlson, F.J.; Coffeng, L.E.; Dandona, L.; Erskine, H.E.; et al. Global and national burden of diseases and injuries among children and adolescents between 1990 and 2013: Findings from the global burden of disease 2013 study. JAMA Pediatr. 2016, 170, 267–287. [Google Scholar] [PubMed]
- World Health Organization; Centers for Disease Control and Prevention. Assessing the Iron Status of Populations: Including Literature Reviews; Report of a Joint World Health Organization/Centers for Disease Control and Prevention Technical Consultation on the Assessment of Iron Status at the Population Level; World Health Organization, Centers for Disease Control and Prevention: Geneva, Switzerland, 2007. [Google Scholar]
- Haas, J.D.; Brownlie, T.T. Iron deficiency and reduced work capacity: A critical review of the research to determine a causal relationship. J. Nutr. 2001, 131, 676S–688S. [Google Scholar] [PubMed]
- Lozoff, B.; Beard, J.; Connor, J.; Barbara, F.; Georgieff, M.; Schallert, T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr. Rev. 2006, 64, S34–S43. [Google Scholar] [CrossRef] [PubMed]
- Ezzati, M.; Lopez, A.; Rodgers, A.; Murray, C.J.L. (Eds.) Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors; World Health Organization: Geneva, Switzerland, 2004.
- Stoltzfus, R.J.; Dreyfuss, M.L. Guidelines for the Use of Iron Supplements to Prevent and Treat Iron Deficiency Anemia; World Health Organization, International Nutritional Anemia Consultative Group: Geneva, Switzerland, 1998. [Google Scholar]
- Raiten, D.J.; Ashour, F.A.S.; Ross, A.C.; Meydani, S.N.; Dawson, H.D.; Stephensen, C.B.; Brabin, B.J.; Suchdev, P.S.; van Ommen, B.; Group, I.C. Inflammation and nutritional science for programs/policies and interpretation of research evidence (inspire). J. Nutr. 2015, 145, 1039S–1108S. [Google Scholar] [CrossRef] [PubMed]
- Best, C.; Neufingerl, N.; van Geel, L.; van den Briel, T.; Osendarp, S. The nutritional status of school-aged children: Why should we care? Food Nutr. Bull. 2010, 31, 400–417. [Google Scholar] [CrossRef] [PubMed]
- Suchdev, P.S.; Namaste, S.M.; Aaron, G.J.; Raiten, D.J.; Brown, K.H.; Flores-Ayala, R.; Group, B.W. Overview of the biomarkers reflecting inflammation and nutritional determinants of anemia (brinda) project. Adv. Nutr. 2016, 7, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Olaiz-Fernández, G.; Rivera-Dommarco, J.; Shamah-Levy, T.; Rojas, R.; Villalpando-Hernández, S.; Hernández-Avila, M.; Sepúlveda-Amor, J. Encuesta Nacional de Salud y Nutrición 2006; Instituto Nacional de Salud Pública: Cuernavaca, México, 2006. [Google Scholar]
- Centers for Disease Control and Prevention; National Center for Health Statistics. National Health and Nutrition Examination Survey Data; US Department of Health and Human Services, Centers for Disease Control and Prevention: Hyattsville, MD, USA, 2003–2006.
- Centers for Disease Control and Prevention; National Center for Health Statistics. National Health and Nutrition Examination Survey Questionnaire (or Examination Protocol, or Laboratory Protocol); US Department of Health and Human Services, Centers for Disease Control and Prevention: Hyattsville, MD, USA, 2003–2006.
- Colombian Family Welfare Institute, Ministry of Social Protection (Colombia), National Institute of Health (Colombia), Profamilia. Colombia National Survey of the Nutritional Situation 2010. Available online: http://ghdx.healthdata.org/record/colombia-national-survey-nutritional-situation-2010 (accessed on 1 July 2015).
- Sullivan, K.M.; Mei, Z.; Grummer-Strawn, L.; Parvanta, I. Haemoglobin adjustments to define anaemia. Trop. Med. Int. Health TMIH 2008, 13, 1267–1271. [Google Scholar] [CrossRef] [PubMed]
- Pardo, C.; Pineros, M.; Jones, N.R.; Warren, C.W. Results of global youth tobacco surveys in public schools in Bogota, Colombia. J. Sch. Health 2010, 80, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Pardo, C.; Pineros, M. Teenage tobacco consumption in five Colombian cities. Biomed. Rev. Inst. Natl. Salud 2010, 30, 509–518. [Google Scholar]
- Howe, L.D.; Galobardes, B.; Matijasevich, A.; Gordon, D.; Johnston, D.; Onwujekwe, O.; Patel, R.; Webb, E.A.; Lawlor, D.A.; Hargreaves, J.R. Measuring socio-economic position for epidemiological studies in low- and middle-income countries: A methods of measurement in epidemiology paper. Int. J. Epidemiol. 2012, 41, 871–886. [Google Scholar] [CrossRef] [PubMed]
- Unites States Census Bureau. Available online: https://www.census.gov/topics/income-poverty/poverty.html#ratio of income to poverty (accessed on 1 July 2015).
- Chumlea, W.C.; Schubert, C.M.; Roche, A.F.; Kulin, H.E.; Lee, P.A.; Himes, J.H.; Sun, S.S. Age at menarche and racial comparisons in US girls. Pediatrics 2003, 111, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Oberg, A.S.; Villamor, E. Low digit ratio predicts early age at menarche in Colombian schoolgirls. Paediatr. Perinat. Epidemiol. 2012, 26, 448–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ireton, M.J.; Carrillo, J.C.; Caro, L.E. Biometry and sexual maturity in a sample of Colombian schoolchildren from El Yopal. Ann. Hum. Biol. 2011, 38, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Marvan, M.L.; Alcala-Herrera, V. Age at menarche, reactions to menarche and attitudes towards menstruation among Mexican adolescent girls. J. Pediatr. Adolesc. Gynecol. 2014, 27, 61–66. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Serum Ferritin Concentrations for the Assessment of Iron Status and Iron Deficiency in Populations; WHO reference number: WHO/NMH/NHD/EPG/112; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- World Health Organization. Serum Transferrin Receptor Levels for the Assessment of Iron Status and Iron Deficiency in Populations; WHO reference number: WHO/NMH/NHD/EPG/146; WTO: Geneva, Switzerland, 2014. [Google Scholar]
- Grant, F.K.; Suchdev, P.S.; Flores-Ayala, R.; Cole, C.R.; Ramakrishnan, U.; Ruth, L.J.; Martorell, R. Correcting for inflammation changes estimates of iron deficiency among rural Kenyan preschool children. J. Nutr. 2012, 142, 105–111. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Serum Retinol Concentrations for Determining the Prevalence of Vitamin a Deficiency in Populations; WHO reference number: WHO/NMH/NHD/MNM/113; WTO: Geneva, Switzerland, 2011. [Google Scholar]
- World Health Organization. C-Reactive Protein Concentrations as a Marker of Inflammation or Infection for Interpreting Biomarkers of Micronutrient Status; WHO reference number: WHO/NMH/NHD/EPG/147; WTO: Geneva, Switzerland, 2014. [Google Scholar]
- Guyatt, G.H.; Oxman, A.D.; Ali, M.; Willan, A.; McIlroy, W.; Patterson, C. Laboratory diagnosis of iron-deficiency anemia: An overview. J. Gen. Intern. Med. 1992, 7, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Thurnham, D.I.; Northrop-Clewes, C.A.; Knowles, J. The use of adjustment factors to address the impact of inflammation on vitamin a and iron status in humans. J. Nutr. 2015, 145, 1137S–1143S. [Google Scholar] [CrossRef] [PubMed]
- Engle-Stone, R.; Haskell, M.J.; Ndjebayi, A.O.; Nankap, M.; Erhardt, J.G.; Gimou, M.M.; Brown, K.H. Plasma retinol-binding protein predicts plasma retinol concentration in both infected and uninfected Cameroonian women and children. J. Nutr. 2011, 141, 2233–2241. [Google Scholar] [CrossRef] [PubMed]
- Villalpando, S.; Shamah-Levy, T.; Garcia-Guerra, A.; Mundo-Rosas, V.; Dominguez, C.; Mejia-Rodriguez, F. The prevalence of anemia decreased in Mexican preschool and school-age children from 1999 to 2006. Salud Publica Mex. 2009, 51, S507–S514. [Google Scholar] [CrossRef] [PubMed]
- Hudson-Thomas, M.; Bingham, K.C.; Simmons, W.K. An evaluation of the hemocue for measuring haemoglobin in field studies in Jamaica. Bull. World Health Organ. 1994, 72, 423–426. [Google Scholar] [PubMed]
- Johns, W.L.; Lewis, S.M. Primary health screening by haemoglobinometry in a tropical community. Bull. World Health Organ. 1989, 67, 627–633. [Google Scholar] [PubMed]
- Sarmiento, O.L.; Parra, D.C.; Gonzalez, S.A.; Gonzalez-Casanova, I.; Forero, A.Y.; Garcia, J. The dual burden of malnutrition in Colombia. Am. J. Clin. Nutr. 2014, 100, 1628S–1635S. [Google Scholar] [CrossRef] [PubMed]
- De Onis, M.; Onyango, A.W.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a who growth reference for school-aged children and adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Shamah-Levy, T.; Villalpando, S.; Jauregui, A.; Rivera, J.A. Overview of the nutritional status of selected micronutrients in Mexican children in 2006. Salud Publica Mex. 2012, 54, 146–151. [Google Scholar] [PubMed]
- Ramirez-Velez, R.; Matinez-Torres, J.; Meneses-Echavez, J.F. Prevalence and demographic factors associated with ferritin deficiency in Colombian children, 2010. Rev. Peru. Med. Exp. Salud Publica 2014, 31, 237–242. [Google Scholar] [PubMed]
- Cogswell, M.E.; Looker, A.C.; Pfeiffer, C.M.; Cook, J.D.; Lacher, D.A.; Beard, J.L.; Lynch, S.R.; Grummer-Strawn, L.M. Assessment of iron deficiency in US preschool children and nonpregnant females of childbearing age: National health and nutrition examination survey 2003–2006. Am. J. Clin. Nutr. 2009, 89, 1334–1342. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, L.L.; Delnatus, J.R.; Odom, A.R.; Eaton, J.C.; Griggs, J.J.; Brown, S.; Wolff, P.B. Determinants of anemia and hemoglobin concentration in haitian school-aged children. Am. J. Trop. Med. Hyg. 2015, 93, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Mesfin, F.; Berhane, Y.; Worku, A. Anemia among primary school children in Eastern Ethiopia. PLoS ONE 2015, 10, e0123615. [Google Scholar] [CrossRef] [PubMed]
- Assefa, S.; Mossie, A.; Hamza, L. Prevalence and severity of anemia among school children in Jimma town, Southwest Ethiopia. BMC Hematol. 2014, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Country and Lending Groups—The World Bank. Available online: http://data.worldbank.org/about/country-and-lending-groups (accessed on 20 November 2015).
- Victora, C.G.; Rivera, J.A. Optimal child growth and the double burden of malnutrition: Research and programmatic implications. Am. J. Clin. Nutr. 2014, 100, 1611S–1612S. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic; Report of a who consultation; WHO Technical Report Series, No. 894; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Tzioumis, E.; Adair, L.S. Childhood dual burden of under- and overnutrition in low- and middle-income countries: A critical review. Food Nutr. Bull. 2014, 35, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Uauy, R.; Garmendia, M.L.; Corvalan, C. Addressing the double burden of malnutrition with a common agenda. Nestle Nutr. Inst. Workshop Ser. 2014, 78, 39–52. [Google Scholar] [PubMed]
- Kroker-Lobos, M.F.; Pedroza-Tobias, A.; Pedraza, L.S.; Rivera, J.A. The double burden of undernutrition and excess body weight in Mexico. Am. J. Clin. Nutr. 2014, 100, 1652S–1658S. [Google Scholar] [CrossRef] [PubMed]
- Rivera, J.A.; Pedraza, L.S.; Martorell, R.; Gil, A. Introduction to the double burden of undernutrition and excess weight in Latin America. Am. J. Clin. Nutr. 2014, 100, 1613S–1616S. [Google Scholar] [CrossRef] [PubMed]
- Iriart, C.; Boursaw, B.; Rodrigues, G.P.; Handal, A.J. Obesity and malnutrition among hispanic children in the United States: Double burden on health inequities. Rev. Panam. Salud Publica 2013, 34, 235–243. [Google Scholar] [PubMed]
- Pasricha, S.R.; Black, J.; Muthayya, S.; Shet, A.; Bhat, V.; Nagaraj, S.; Prashanth, N.S.; Sudarshan, H.; Biggs, B.A.; Shet, A.S. Determinants of anemia among young children in rural India. Pediatrics 2010, 126, e140–e149. [Google Scholar] [CrossRef] [PubMed]
- Foote, E.M.; Sullivan, K.M.; Ruth, L.J.; Oremo, J.; Sadumah, I.; Williams, T.N.; Suchdev, P.S. Determinants of anemia among preschool children in rural, Western Kenya. Am. J. Trop. Med. Hyg. 2013, 88, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Righetti, A.A.; Koua, A.Y.; Adiossan, L.G.; Glinz, D.; Hurrell, R.F.; N’Goran, E.K.; Niamke, S.; Wegmuller, R.; Utzinger, J. Etiology of anemia among infants, school-aged children, and young non-pregnant women in different settings of south-central Cote d’ivoire. Am. J. Trop. Med. Hyg. 2012, 87, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Leenstra, T.; Kariuki, S.K.; Kurtis, J.D.; Oloo, A.J.; Kager, P.A.; ter Kuile, F.O. Prevalence and severity of anemia and iron deficiency: Cross-sectional studies in adolescent schoolgirls in Western Kenya. Eur. J. Clin. Nutr. 2004, 58, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Brito, L.L.; Barreto, M.L.; Rde, C.S.; Assis, A.M.; Reis, M.G.; Parraga, I.M.; Blanton, R.E. Moderate- and low-intensity co-infections by intestinal helminths and schistosoma mansoni, dietary iron intake, and anemia in Brazilian children. Am. J. Trop. Med. Hyg. 2006, 75, 939–944. [Google Scholar] [PubMed]
- Szarfarc, S.C.; de Souza, S.B. Prevalence and risk factors in iron deficiency and anemia. Arch. Latinoam. Nutr. 1997, 47, 35–38. [Google Scholar] [PubMed]
- Shirasawa, T.; Ochiai, H.; Nanri, H.; Nishimura, R.; Ohtsu, T.; Hoshino, H.; Tajima, N.; Kokaze, A. Trends of underweight and overweight/obesity among japanese schoolchildren from 2003 to 2012, defined by body mass index and percentage overweight cutoffs. J. Epidemiol. Jpn. Epidemiol. Assoc. 2015, 25, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Lee, H.J.; Jang, H.B.; Park, J.Y.; Kang, J.H.; Park, K.H.; Song, J. Effects of maternal education on diet, anemia, and iron deficiency in Korean school-aged children. BMC Public Health 2011, 11, 870. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.L.; Myers, J.E.; Kriebel, D. Prevalence odds ratio or prevalence ratio in the analysis of cross sectional data: What is to be done? Occup. Environ. Med. 1998, 55, 272–277. [Google Scholar] [CrossRef] [PubMed]
- National Health And Nutrition Examination Survey 2003–2004 Lab Methods: Complete Blood Count. Available online: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/l25_c_met_complete_blood_count.pdf (accessed on 11 November 2015).
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity; (WHO/NMH/NHD/MNM/11.1); Vitamin and Mineral Nutrition Information System, World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Wieringa, F.T.; Dijkhuizen, M.A.; West, C.E.; Northrop-Clewes, C.A.; Muhilal. Estimation of the effect of the acute phase response on indicators of micronutrient status in Indonesian infants. J. Nutr. 2002, 132, 3061–3066. [Google Scholar] [PubMed]
- Northrop-Clewes, C.A. Interpreting indicators of iron status during an acute phase response—Lessons from malaria and human immunodeficiency virus. Ann. Clin. Biochem. 2008, 45, 18–32. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Mexico/n = 3660 | USA/n = 733 | Colombia/n = 8573 | |||
|---|---|---|---|---|---|---|
| n * | % (SE of %) or Mean (SE) | n * | % (SE of %) or Mean (SE) | n * | % (SE of %) or Mean (SE) | |
| Demographics | ||||||
| Age in years | 3660 | 9.1 (0.1) | 733 | 13.6 (0.04) | 8573 | 9.9 (0.04) |
| Age | ||||||
| <12.0 years | 3083/3660 | 78.7% (1.2) | - | - | 6223/8573 | 72.5% (0.6) |
| 12.0–14.99 years | 577/3660 | 21.3% (1.2) | 733/733 | 100% (0.0) | 2350/8573 | 27.5% (0.6) |
| Sex (Females) % | 2115/3660 | 59.5% (1.6) | 733 | 100% (0.0) | 4944/8573 | 57.4% (0.7) |
| Race/Ethnicity | ||||||
| Black | N/A | N/A | 244/733 | 15.6% (2.1) | 958/8573 | 11.6% (0.5) |
| Non-Black | N/A | N/A | 489/733 | 84.4% (2.0) | 7615/8573 | 88.4% (0.5) |
| Asset | ||||||
| Poorest | 1191/3656 | 31.1% (1.8) | 160/717 | 14.3% (1.8) | 3299/8573 | 25.9% (0.8) |
| All Other | 2465/3656 | 68.9% (1.8) | 557/717 | 85.7% (1.8) | 5274/8573 | 74.0% (0.8) |
| Nutrition/Growth | ||||||
| Stunting % (HAZ < −2) | 396/3658 | 10.2% (0.9) | 8/725 | 1.6% (0.7) | 963/8390 | 9.8% (0.5) |
| Wasting % (BAZ < −2) | 54/3658 | 1.3% (0.3) | 8/725 | 2.4% (1.2) | 160/8390 | 2.0% (0.2) |
| Overweight % (BAZ > 2) | 450/3658 | 13.3% (1.3) | 169/725 | 19.9% (2.9) | 339/8390 | 4.3% (0.3) |
| Obese % (BAZ > 3) | 106/3658 | 3.9% (0.8) | 36/725 | 3.6% (0.8) | 40/8390 | 0.5% (0.1) |
| Biochemical markers | ||||||
| Hemoglobin (g/dL) | 3660 | 13.8 (0.6) | 733 | 13.7 (0.7) | 8573 | 14.5 (0.3) |
| 1 Hemoglobin (g/dL) adjusted for altitude and/or 2 smoking | 3660 | 13.3 (0.5) | N/A | N/A | 8573 | 14.1 (0.3) |
| C-Reactive Protein (ng/mL) | 3660 | 2.2 (0.2) | 733 | 1.7 (0.4) | 8573 | 2.5 (0.1) |
| 2 Iron Deficiency % | 626/3302 | 18.1% (1.2) | ‡ 87/680 | 9.7% (1.3) | 736/7450 | 9.2% (0.4) |
| 3 Low Ferritin % | 663/3650 | 17.3% (1.2) | ‡ 92/733 | 9.3% (1.3) | 831/8573 | 9.2% (0.4) |
| 4 High sTFR % | 664/3645 | 19.6% (1.3) | ‡ 49/727 | 5.0% (1.0) | N/A | N/A |
| 5 Vitamin A Deficiency % | N/A | N/A | ‡ 2/720 | 0.1% (0.1) | N/A | N/A |
| 6 Elevated C-Reactive Protein (ng/mL) % | 353/3660 | 9.4% (0.8) | 53/733 | 6.9% (1.2) | 1123/8573 | 13.2% (0.6) |
| 7 Anemia % | 454/3660 | 11.6% (0.9) | 49/733 | 3.6% (0.8) | 452/8573 | 4.2% (0.3) |
| 8 Iron Deficiency Anemia using adjusted Hb % | 103/3302 | 2.6% (0.4) | ‡ 16/680 | 1.4% (0.5) | 77/7450 | 0.7% (0.1) |
| 9 Anemia associated with Iron Deficiency % | - | 22.4% | - | 38.9% | - | 16.7% |
| Characteristics | Mexico | Colombia | ||||||
|---|---|---|---|---|---|---|---|---|
| Anemia (%) * | UnAdj (95% CI) | ** Adj OR (95% CI) | p-Value ‡ | Anemia (%) * | UnAdj (95% CI) | ** Adj OR (95% CI) | p-Value ‡ | |
| Continuous | ||||||||
| 1 CRP (ng/mL) | - | - | 1.0 (0.9–1.0) | 0.16 | - | - | 1.0 (0.99–1.01) | 0.23 |
| Categorical | ||||||||
| 2 Age (years) | ||||||||
| <12.0 years | 12.0 | 4.4 | ||||||
| 12.0–14.99 years | 10.1 | 0.8 (0.5–1.3) | 0.7 (0.5–1.2) | 0.19 | 3.7 | 0.8 (0.6–1.1) | 0.8 (0.5–1.0) | 0.08 |
| Sex | ||||||||
| Male | 11.0 | 4.3 | ||||||
| Female | 12.2 | 1.1 (0.8–1.6) | 1.2 (0.9–1.7) | 0.30 | 4.1 | 1.0 (0.8–1.3) | 1.0 (0.7–1.3) | 0.90 |
| 3 Race/Ethnicity | ||||||||
| Non Black | 3.8 | |||||||
| Black | N/A | N/A | N/A | N/A | 7.1 | 1.9 (0.4–2.7) | 1.6 (1.2–2.3) | 0.005 |
| 4 Asset | ||||||||
| All Other | 11.1 | 3.3 | ||||||
| Poorest | 13.0 | 1.2 (0.9–1.7) | 1.1 (0.8–1.6) | 0.55 | 6.7 | 2.1 (1.6–2.9) | 1.8 (1.3–2.5) | 0.0005 |
| 5 Low Ferritin % | ||||||||
| No | 10.9 | 3.7 | ||||||
| Yes | 14.8 | 1.4 (1.0–2.0) | 1.5 (1.1–2.0) | 0.02 | 9.3 | 2.7 (1.9–3.7) | 2.7 (2.0–3.8) | <0.0001 |
| 6 Overweight | ||||||||
| No | 12.6 | 4.3 | ||||||
| Yes | 5.5 | 0.4 (0.2–0.8) | 0.4 (0.2–0.8) | 0.007 | 1.7 | 0.4 (0.2–1.0) | 0.5 (0.2–1.2) | 0.11 |
| 7 Stunting | ||||||||
| No | 11.7 | 3.9 | ||||||
| Yes | 11.6 | 1.0 (0.6–1.5) | 0.9 (0.5–1.4) | 0.51 | 6.3 | 1.7 (1.1–2.4) | 1.6 (1.1–2.3) | 0.02 |
| † Characteristics | USA | |||
|---|---|---|---|---|
| Anemia (%) * | UnAdj (95% CI) | ** Adj OR (95% CI) | p-Value ‡ | |
| Continuous | ||||
| 1 CRP (ng/mL) | - | - | 1.0 (0.99–1.04) | 0.06 |
| 2 Age (years) | - | - | 0.9 (0.4–1.8) | 0.72 |
| Categorical | ||||
| 3 Race/Ethnicity | ||||
| Non Black | 1.2 | |||
| Black | 16.7 | 16.5 (5.5–49.5) | 14.1 (4.7–42.1) | <0.0001 |
| 4 Asset | ||||
| All Other | 3.0 | |||
| Poorest | 7.9 | 2.8 (1.3–5.9) | 1.3 (0.8–2.2) | 0.33 |
| 5 Low Ferritin % | ||||
| No | 2.2 | |||
| Yes | 17.2 | 9.1 (4.5–18.4) | 8.0 (3.0–21.3) | <0.0001 |
| 6 Overweight | ||||
| No | 3.6 | |||
| Yes | 3.9 | 1.1 (0.4–3.2) | 0.8 (0.3–2.0) | 0.63 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syed, S.; Addo, O.Y.; De la Cruz-Góngora, V.; Ashour, F.A.S.; Ziegler, T.R.; Suchdev, P.S. Determinants of Anemia among School-Aged Children in Mexico, the United States and Colombia. Nutrients 2016, 8, 387. https://doi.org/10.3390/nu8070387
Syed S, Addo OY, De la Cruz-Góngora V, Ashour FAS, Ziegler TR, Suchdev PS. Determinants of Anemia among School-Aged Children in Mexico, the United States and Colombia. Nutrients. 2016; 8(7):387. https://doi.org/10.3390/nu8070387
Chicago/Turabian StyleSyed, Sana, O. Yaw Addo, Vanessa De la Cruz-Góngora, Fayrouz A. Sakr Ashour, Thomas R. Ziegler, and Parminder S. Suchdev. 2016. "Determinants of Anemia among School-Aged Children in Mexico, the United States and Colombia" Nutrients 8, no. 7: 387. https://doi.org/10.3390/nu8070387