Monarch Butterflies Show Differential Utilization of Nine Midwestern Milkweed Species
- 1Department of Entomology, The Pennsylvania State University, University Park, PA, United States
- 2Department of Ecology, Evolution and Organismal Biology, Iowa State University, Ames, IA, United States
- 3Department of Ecology, Montana State University, Bozeman, MT, United States
- 4Corn Insects and Crop Genetics Research Unit, United States Department of Agriculture, Agricultural Research Station, Department of Entomology, Iowa State University, Ames, IA, United States
- 5Departments of Natural Resource Ecology and Management and Entomology, Iowa State University, Ames, IA, United States
Monarch butterfly overwintering numbers have declined over the past 20 years. Restoring habitat that includes milkweeds, the only host plants for larval monarch butterflies, is necessary to increase monarch numbers within the breeding range. The value of different milkweed species for restoration will depend, in part, on the extent to which they are utilized by ovipositing females. The number of eggs laid on different species over a season will be a function of plant size and phenology as well as female preference. We examined seasonal egg deposition and females' oviposition choices by comparing the number of eggs laid by free-flying wild monarchs on each of nine native milkweed species occurring in Iowa (Asclepias syriaca, Asclepias tuberosa, Asclepias incarnata, Asclepias verticillata, Asclepias exaltata, Asclepias hirtella, Asclepias speciosa, Asclepias sullivantii, and Cynanchum laeve). One plot, consisting of clusters of each of the nine species, was established at each of 14 sites across the state of Iowa. Eggs were counted weekly in June, July and August 2015–2017. The highest egg totals were recorded on A. incarnata and A. syriaca in all years. Fewer eggs were counted on A. exaltata, A. hirtella, A. tuberosa, A. verticillata, and C. laeve. Our results show that monarchs prefer some milkweed species over others, but that they can use all nine native milkweed species for oviposition.
Introduction
Habitat loss is one of the leading causes of species decline for many taxa, (Means and Simberloff, 1987; Wilcove et al., 1998; Pimm and Raven, 2000; Ceballos and Ehrlich, 2002,?; Kerr and Cihlar, 2004; Venter et al., 2006; Xu et al., 2018). Over the past 20 years, monarch populations have experienced a significant decline in overwintering numbers (Brower et al., 2012; Espeset et al., 2016; Inamine et al., 2016; Schultz et al., 2017). Loss of milkweed within the breeding range is considered by many scientists to be the leading cause of the decline of the monarch population east of the Rocky Mountains (Pleasants and Oberhauser, 2013; Flockhart et al., 2015; Pleasants, 2017; Zaya et al., 2017). Restoration of Midwestern monarch habitat is essential to increase (population numbers Oberhauser et al., 2016 as many of the monarchs that overwinter in Mexico originate from this area Wassenaar and Hobson, 1998; Flockhart et al., 2017. Organizations federal, state, and non-profit) have started efforts to establish monarch habitat, especially adding milkweeds to the landscape in critical land cover/land use categories to enhance monarch reproduction (Thogmartin et al., 2017).
Knowledge of what species to include in habitat restoration is necessary to develop and implement an effective conservation program. Although monarch butterflies (Danaus plexippus) are dependent upon milkweeds (Asclepias spp.) as larvae, there are over 100 species of milkweeds in the U.S. (Woodson, 1954) and we need to know how available each species is throughout the season, which ones are better for larval growth and on which ones monarch females choose to lay eggs. Currently, the majority of monarchs in population east of the Rocky Mountains feed on Asclepias syriaca in the summer (Wassenaar and Hobson, 1998). Rather than reflecting a preference, this may be because disturbance from modern agricultural has made A. syriaca the dominant species on the landscape (Martin and Burnside, 1980). This species may not have been as prevalent in historic landscapes (Hayden, 1919; Pleasants, 2015). More information is needed about monarch butterflies' use of other native milkweed species both as larvae and adults beyond A. syriaca, the milkweed on which all current conservation recommendations are based (Landis, 2013; Pleasants and Oberhauser, 2013; Pleasants, 2017).
Prior work has contributed to our understanding of monarchs' oviposition choices and use of different milkweed species (Cohen and Brower, 1982; Malcolm et al., 1989; Zalucki et al., 1990; Haribal and Renwick, 1996, 1998a,b; Calvert, 1999; Bartholomew and Yeargan, 2002; DiTommaso and Losey, 2003; Ladner and Altizer, 2005; Casagrande and Dacey, 2007) as well as larval survival on different species (Cohen and Brower, 1982; Zalucki et al., 1990; Zalucki and Brower, 1992; Ladner and Altizer, 2005; Yeargan and Allard, 2005; Robertson et al., 2015; Baker and Potter, 2018). These studies have not compared larval survival and oviposition preference patterns across the same set of co-occurring milkweed species in both laboratory and field settings.
In our prior work (Pocius et al., 2017b, 2018), we compared larval survival on nine milkweed species and oviposition preference on four of these species in a laboratory setting. These nine species are native to Iowa, which is a high priority area for Midwestern conservation efforts (Flockhart et al., 2015; Thogmartin et al., 2017). Most milkweed species native to the Midwest have not been evaluated in field experiments. The species we tested included: A. syriaca (common milkweed), Asclepias incarnata (swamp milkweed), Asclepias tuberosa (butterfly milkweed), Asclepias verticillata (whorled milkweed), Asclepias speciosa (showy milkweed), Asclepias exaltata (poke milkweed), Asclepias sullivantii (prairie milkweed), Asclepias hirtella (tall green milkweed), and Cynanchum laeve (honeyvine milkweed). These milkweeds have overlapping ranges (Woodson, 1954; Kaul et al., 1991; Eilers and Roosa, 1994), but varying habitat needs as well as differing concentrations of phytochemicals including cardenolides (Roeske et al., 1976; Malcolm, 1991; Rasmann and Agrawal, 2011); and quercetin glycosides (Haribal and Renwick, 1996). The species also have different plant architecture (stem height, leaf width, leaf shape, stem branching, etc.; Woodson, 1954).
Our laboratory results suggest that monarch larvae will consume, survive, and eventually pupate on all nine Midwestern milkweed species (Pocius et al., 2017b); however, fewer larvae reached adulthood when they fed on A. hirtella and A. sullivantii (Pocius et al., 2017b, see Table 1). Larval survival was not significantly different among the other seven milkweed species; these species may provide equal benefits for larvae when included in habitat restorations within the native range of each milkweed species (Pocius et al., 2017b). Our laboratory oviposition results, using just A. incarnata, A. syriaca, A. tuberosa, and A. verticillata, suggest that monarch butterflies prefer to oviposit on A. incarnata and A. syriaca although they will utilize all four species (Pocius et al., 2018-see also Baker and Potter, 2018). Here, we build on prior laboratory work with a report of field oviposition using all nine milkweed species. We compare the total number of eggs laid on each species in June and July, in 2015 and 2016, to compare females' choices when all nine species were present, before senescence of three of the species. We also compared the total number of eggs laid on each of six species present in July and August, in 2015 and 2016, to capture females' choices during peak oviposition. Finally, we compare the total number of eggs laid on each species during the entire summer season, in 2015–2017, to provide estimates of monarch utilization of each milkweed species for habitat restoration purposes.
Materials and Methods
Field Oviposition
Experimental Milkweed Plots and Site Establishment
Midwestern ecotype milkweed seeds of A. exaltata, A. hirtella, A. incarnata, A. speciosa, A. sullivantii, A. syriaca, A. tuberosa, A. verticillata, and C. laeve (Prairie Moon Nursery, MN, USA) were stratified in wet sand for 6 weeks. After stratification, seeds were sown into 128-cell plug trays (Landmark Plastics, Akron OH, USA) and transplanted into 8.9 cm2, deep perennial pots (Kord, Ontario Canada) at approximately 6 weeks following germination. When milkweed plants were 12 weeks old, five young plants of each species were transported to each location. Sites were established at ten Iowa State Research and Demonstration Farms (Newell, IA; Lewis, IA; Boone, IA; Ames, IA; Chariton, IA; Nashua, IA; Kanawha, IA; Sutherland, IA; and Castana, IA), Luther College (Decorah, IA), Pella High School (Pella, IA), Central College (Pella, IA), and on the Sorenson-Powell property (Adel, IA). At least one site was located in each quadrant of the state. Plants were distributed to each site and planted by the second week of June 2015.
Each of nine milkweed species was randomly assigned to a 1 m2 plot within a single row at each site. Each plot consisted of 5 plants for a total of 45 plants at each site. Plots were separated from each other by a 1 m wide grass or stone path. Any plants that did not survive were replaced with young plants (6–8 weeks old) twice during the summer of 2015 and at the beginning of the season in 2016. A. hirtella plants were not replaced due to a lack of seed in 2016 and 2017.
Site Monitoring
Each site was monitored weekly from the first week of June 2015 through the end of August 2017 for a total of 42 visits to each site. Each week, the number of live milkweed plants, bloom presence, the number of blooms, the height of the tallest plant, the presence of seed pods, and the presence of mature seed pods was recorded for each milkweed species. Each plant was examined for the presence of monarch eggs, larvae, or other insects using a modified protocol from the Monarch Larva Monitoring Project (Oberhauser, 2013).
Statistical Analysis
The total number of eggs on each plot of five plants was summed across June, July, and August for each site and then averaged; the results were analyzed separately for each year. Only sites where observers recorded egg numbers for at least 8 weeks were included in the analysis of each year. Sites without any eggs during the summer within each year were removed from the analysis (N = 12 sites in 2015, N = 13 sites in 2016, and N = 10 sites in 2017). Egg counts were only reported for milkweed species with live plants at each site over the observation period. Differences in total egg counts in single years were determined using a Poisson regression with milkweed species (Pocius et al., 2018) as a fixed effect and site a random effect. Pairwise differences in egg counts were determined by comparing least square means for each milkweed species (Pocius et al., 2018); p-values were adjusted using Tukey's range test for multiple comparisons (Pocius et al., 2018). Concordance was determined using a Kendall coefficient of concordance. Correlation between average egg counts and average plant traits were determined using a Pearson correlation. R version 3.3.3 (R Core Team, 2014) was used for all statistical analyses.
To address preference directly, the total number of eggs in each plot of five plants were summed across June and July in 2015 and 2016 when all nine species were available and prior to senescence of A. exaltata, A. hirtella, and A. speciosa. The total number of eggs in each plot were also summed across the six milkweed species present in across July and August in 2015 and 2016 to include the timing of peak oviposition in the analysis of these years. The year 2017 was excluded from preference analyses because some species had disappeared from the plots by then. Only sites where eggs were laid were included in the analysis (N = 12 in 2015 and N = 11 in 2016). Differences in total egg counts in each year were determined using a Poisson regression with milkweed species (Pocius et al., 2018) as a fixed effect and site as a random effect. Plant height and bloom count were not significant predictors of the number of eggs laid per species and were excluded from the final model. Pairwise differences in egg counts were determined by comparing least square means for each milkweed species (Pocius et al., 2018); p-values were adjusted using Tukey's range test for multiple comparisons (Pocius et al., 2018).
Results
Field Oviposition
2015
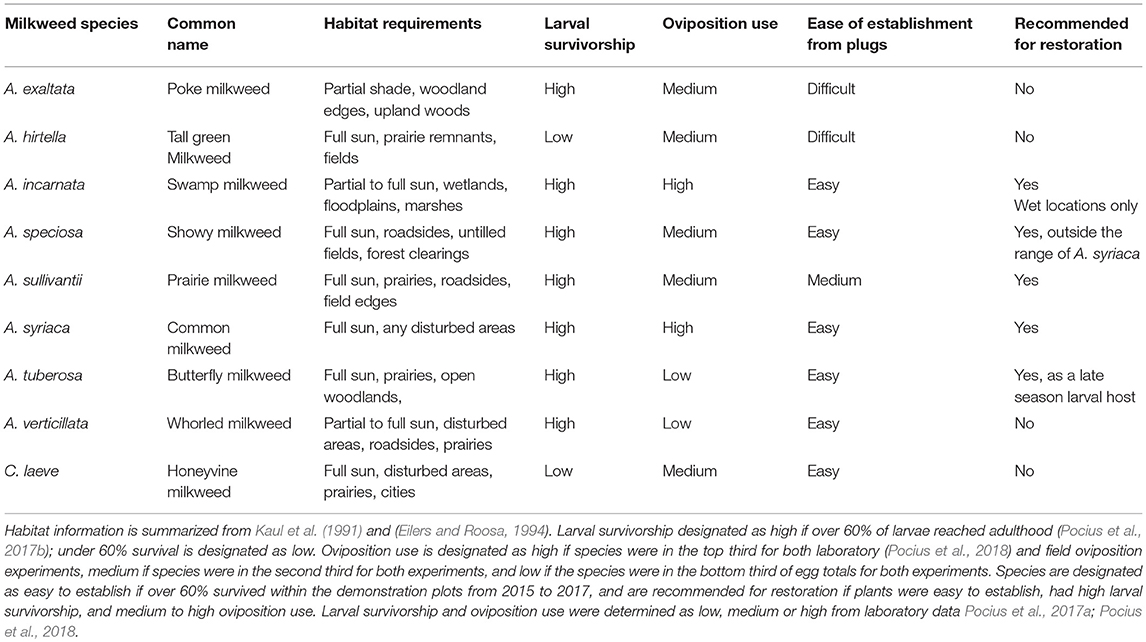
Milkweed species had a significant effect on the total number of eggs laid per milkweed species. A. incarnata had the highest egg totals when counts from all sites were combined across the entire breeding season (Figure 1A). Females laid 1.3 times more eggs on A. incarnata than A. syriaca, although this difference was not significant (z = 2.12, p > 0.4). One of the largest differences in total egg counts was between A. incarnata or A. syriaca and A. exaltata. Females laid 6.8 times more eggs on A. incarnata (z = −4.04, p < 0.001) and 5.4 times more eggs on A. syriaca (z = −6.59, p < 0.001) than on A. exaltata (Figure 1A). All other significant pairwise comparisons are shown in Supplementary Table 1.
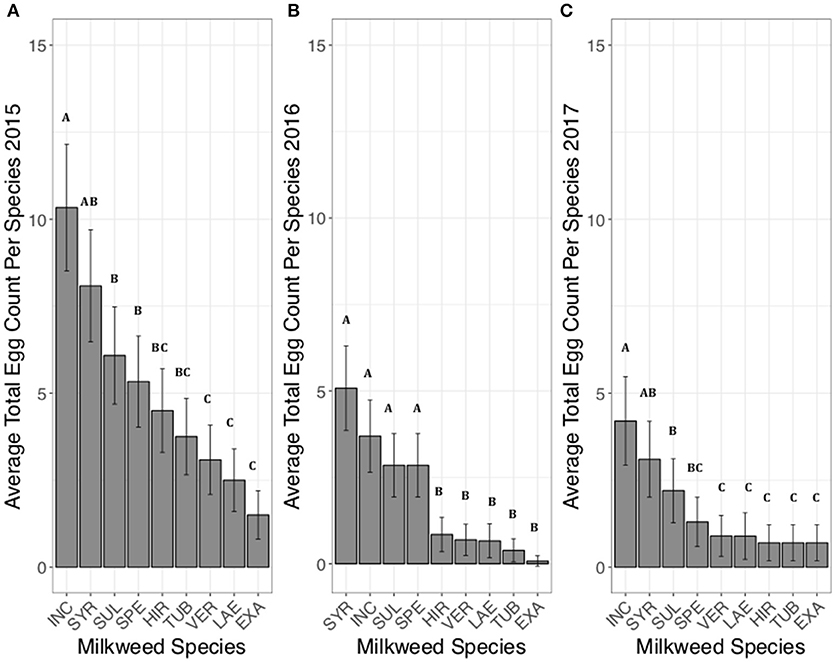
Figure 1. Average eggs counted on each milkweed species over the course of the summer breeding season in 2015 (A), 2016 (B), and 2017 (C). Each bar represents one milkweed species. EXA, A. exaltata; HIR, A. hirtella; INC, A. incarnata; LAE, C. laeve; SPE, A. speciosa; SUL, A. sullivantii; SYR, A. syriaca; TUB, A. tuberosa; VER, A. verticillata; error bars represent 95% confidence intervals. N = 12 sites in 2015, 12 sites in 2016, and 10 sites in 2017. Bars that do not share a letter within each panel are significantly different from each other. Females laid more eggs on A. incarnata and A. syriaca than on A. exaltata, A. hirtella, C. laeve, A. tuberosa, and A. verticillata in all years (p < 0.05). P-values were adjusted using the Tukey method for multiple comparisons.
For June-July, when all species were present, A. incarnata and A. hirtella had the highest average egg totals per site (Figure 2A). A. incarnata had significantly higher egg counts compared to A. exaltata, C. laeve, A. tuberosa, and A. verticillata (Figure 2A, Supplementary Table 2). A. exaltata had significantly lower egg counts than A. hirtella and A. sullivantii (Figure 2A, Supplementary Table 2). The number of eggs laid on A. syriaca was not significantly different from A. incarnata. All other significant pairwise comparisons are shown in Supplementary Table 2.
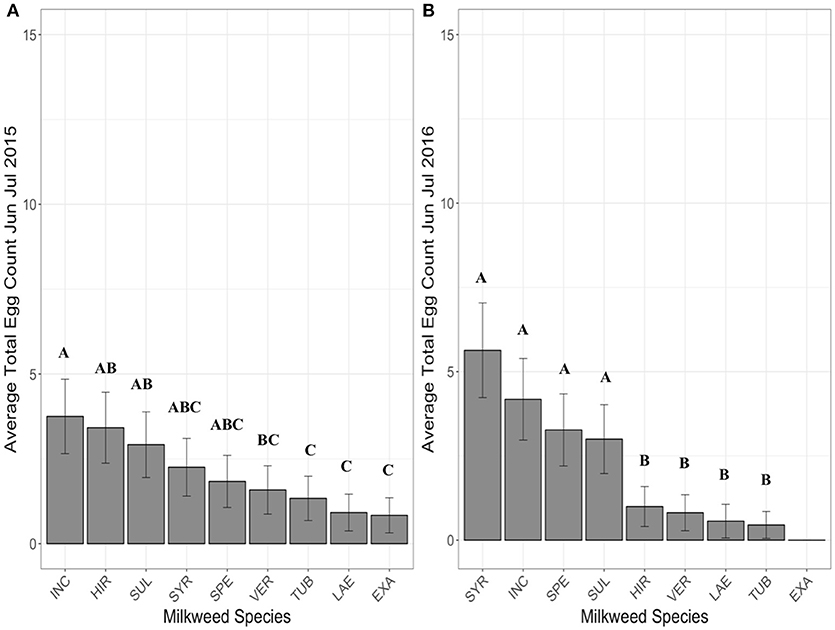
Figure 2. Average eggs counted on each milkweed species in June and July in 2015 (A), 2016 (B) when 5 plants of each species were present in each research plot. Each bar represents one milkweed species. EXA, A. exaltata; HIR, A. hirtella; INC, A. incarnata; LAE, C. laeve; SPE, A. speciosa; SUL, A. sullivantii; SYR, A. syriaca; TUB, A. tuberosa; VER, A. verticillata; error bars represent 95% confidence intervals. N = 11 sites in 2015, and 12 sites in 2016. Bars that share a letter within each panel are not significantly different from each other. Females laid more eggs on A. incarnata than on A. exaltata, C. laeve, A. tuberosa, and A. verticillata in both years (p < 0.05). The number of eggs laid on A. syriaca was not significantly different from A. incarnata. P-values were adjusted using the Tukey method for multiple comparisons.
When the period from July through August was examined, with A. exaltata, A. hirtella, and A. speciosa removed due to senescence, A. incarnata and A. syriaca had the highest average egg totals per site (Figure 3A). The largest differences in egg counts were between A. incarnata and C. laeve (z = 7.02, p < 0.0001), A. tuberosa (z = 5.86, p < 0.0001), and A. verticillata (z = 6.49, p < 0.0001). A. verticillata (z = 5.02, p < 0.0001), A. tuberosa (z = 4.28, p = 0.0003), and C. laeve (z = −5.65, p < 0.0001) also had significantly fewer eggs than A. syriaca although A. sullivantii and A. syriaca were not significantly different from each other. All other significant pairwise comparisons are shown in Supplementary Table 3.
Figure 3. Average eggs counted on each milkweed species in July and August in 2015 (A), 2016 (B) when 5 plants of each species were present in each research plot. Each bar represents one milkweed species. INC, A. incarnata; LAE, C. leave; SUL, A. sullivantii; SYR, A. syriaca; TUB, A. tuberosa; VER, A. verticillata; error bars represent 95% confidence intervals. N = 11 sites in 2015, and 12 sites in 2016. Bars that share a letter within each panel are not significantly different from each other. Females laid more eggs on A. incarnata than on A. exaltata, C. laeve, A. tuberosa, and A. verticillata in all years (p < 0.05). The number of eggs laid on A. syriaca was not significantly different from A. incarnata. P-values were adjusted using the Tukey method for multiple comparisons.
2016
Milkweed species had a significant effect on the total number of eggs laid per milkweed species. A. syriaca had the highest average egg totals followed by A. incarnata (Figure 1B). Females laid 1.4 times more eggs on A. syriaca than A. incarnata although this difference was not significant (z = −1.55, p > 0.8). The largest difference in egg counts was observed between A. syriaca or A. incarnata and A. exaltata. Females laid over twenty times more eggs on A. syriaca (z = −4.21, p < 0.01) and A. incarnata (z = −3.87, p < 0.01) than on A. exaltata in 2016. All other significant pairwise comparisons are shown in Supplementary Table 4.
For June-July, when all species were present, species had a significant effect on the average total number of eggs laid per milkweed species (Figure 2B). No eggs were laid on A. exaltata during this time period (Figure 2B). A. incarnata and A. syriaca had the two highest average egg totals (Figure 2B). A. syriaca had significantly higher egg counts than A. hirtella, C. laeve, A. tuberosa, and A. verticillata (Figure 2B, Supplementary Table 5). A. speciosa and A. sullivantii had comparable egg totals to A. incarnata and A. syriaca (Figure 2B). All other significant pairwise comparisons are shown in Supplementary Table 5.
When the period from July through August was examined, with A. exaltata, A. hirtella, and A. speciosa removed due to senescence, A. incarnata and A. syriaca had the highest average egg totals (Figure 3B), but the largest differences in egg counts were between A. syriaca and C. laeve (z = −5.23, p < 0.0001), A. tuberosa (z = 5.63, p < 0.0001), and A. verticillata (z = 5.68, p < 0.0001). A. verticillata (z = 4.67, p < 0.001), A. tuberosa (z = 4.88, p < 0.001), and C. laeve (z = 4.35, p = 0.0002) also had significantly fewer eggs than A. incarnata. A. incarnata, A. syriaca, and A. sullivantii are not significantly different from each other. All other significant pairwise comparisons are shown in Supplementary Table 6.
2017
Milkweed species had a significant effect on the number of total eggs laid per milkweed species. A. incarnata had the highest egg totals while A. syriaca had the second highest egg counts when eggs from all sites were combined (Figure 1C). Females laid about 1.3 times more eggs on A. incarnata than A. syriaca, although this difference was not significant (z = 1.29, p > 0.9). Females laid eight times more eggs on A. incarnata than on A. exaltata (z = −4.44, p = 0.0003) and six times more eggs on A. incarnata than on A. hirtella (z = −4.44, p = 0.0003) in 2017. All other significant pairwise comparisons are shown in Supplementary Table 7.
Comparison Among Years
During each of the 3 years, over the entire summer season, female monarchs laid eggs on all nine milkweed species but a greater number of eggs were laid on some milkweed species than others (Figure 1). The species order of the number of eggs laid was highly concordant across years (W = 0.94). Across years the overall utilization of each species is summarized in Table 1. There was no significant correlation between the average number of blooms per plant and the average number of eggs per plant (r = 0.18, p = 0.25) or the average number of eggs per plant and species plant height (r = −0.07 to 0.09, p > 0.05). The total number of eggs laid was 542 (41.7 eggs per site) in 2015, 221 (13 eggs per site) in 2016 and 136 (10.5 eggs per site) in 2017. When species were compared during a subset of the summer, species order of preference was moderately concordant between June-July 2015 and 2016 (W = 0.50) and highly concordant between July-August 2015 and 2016 (W = 0.70).
Discussion
The findings of our field-based oviposition preference experiment (June through July counts) were consistent across 2015–2016 and suggest that while monarch butterflies will oviposit on all milkweed species tested, some species consistently received fewer total eggs in the research plots; A. exaltata received few eggs across all years. The species on which females chose to oviposit in the June-July period, A incarnata and A. syriaca, were also preferred in July-August. A. incarnata and A. syriaca also had higher egg totals in the field study by Baker and Potter (2018). These two species were also preferred in the laboratory experiment which also included A. verticillata and A. tuberosa (Pocius et al., 2018). Contrary to Zalucki and Kitching (1982) and Baker and Potter (2018), we did not see an increase in egg counts with plant height within species in any year or an increase in the number of eggs laid with increasing bloom count.
Monarchs from the populations both east and west of the Rocky Mountains also have exhibited the same oviposition choices when exposed to the same array of milkweed species (Ladner and Altizer, 2005). Although monarchs exhibited egg-laying patterns in this study, they did lay eggs on all nine species each year. This indicates that although monarchs make oviposition choices, they do not specialize on a single milkweed species. This is important for a species that encounters different sets of milkweed species on the landscape during its annual cycle (Zhan et al., 2014; Agrawal, 2017).
Interestingly, the species on which larvae performed well and those with high egg totals were not always correlated (Mayhew, 1997, 2001; Berdegué et al., 1998; Gratton and Welter, 1998 see Table 1). For example, both A. tuberosa, and A. verticillata were good larval food sources (Pocius et al., 2017b), but fewer eggs were laid on these species in the lab (Pocius et al., 2018) and in the field. This suggests that the factors that female monarchs use to make egg-laying decisions can be different from those that determine larval success.
We saw more eggs on all species in 2015 than 2016 and 2017. These higher egg totals could be due to the young plant age and smaller stature of first-year plants which made them more attractive (Zalucki and Kitching, 1982). Alternatively, the 2015 observations were reflective of the higher level of egg laying in the Midwest in 2015 as compared to 2016 and 2017 (J. Pleasants pers comm.). Eggs were not present at all sites each year, but no site had zero eggs in 2 consecutive years, demonstrating the variability of egg distribution across Iowa during these 3 years. These differences could be related to varying adult recruitment rates in the spring and subsequent habitat utilization across the state later in the summer.
Across years, fewer monarch eggs were deposited on A. exaltata, A. tuberosa, and C. laeve when compared to A. incarnata and A. syriaca. Both A. exaltata and A. hirtella were difficult to establish in these Iowa sites (Table 1). Only four sites had five live plants of both species by August 2017, but the differences in egg counts are apparent in 2015 and 2016 when each site still had 5 live plants of each species. A. exaltata senesced by late July in all years, before peak oviposition occurred. This is likely the primary explanation for its lower overall egg count. The few eggs that we did observe on A. tuberosa were located on flower buds; however, we saw 4th and 5th instars feeding on this species in August. Older larvae may have moved to these plants from the other milkweed species within the site. Because A. tuberosa was in better condition (greener leaves, no visible senescence) compared to A. incarnata, A. speciosa, and A. syriaca late in the growing season, A. tuberosa may be more valuable as a late-season larval food source than for oviposition in August. The utility of C. laeve may be underestimated in our analysis; we observed more eggs on this species anecdotally in September in central Iowa after plot monitoring across the state ended; data from September were not included here. However, it is unlikely that eggs laid that late will successfully produce adults that migrate to Mexico (Orley Taylor pers comm). There is also inherent variation because of the various locations of the research plots. An examination of these site differences is outside the scope of this study.
Annual and inter-annual variation of temperature and precipitation can affect milkweed quality. High-quality milkweed is essential for both larvae and ovipositing females throughout the breeding season. Because some milkweeds thrive in wet conditions (A. incarnata), and others grow well in drier conditions (A. hirtella and A. tuberosa), specialization on one milkweed species is not a viable strategy for ovipositing female monarchs because plant quality is highly variable across the landscape and the duration of the breeding season. Future work should investigate milkweed phenology, milkweed survival after planting, and monarch use across critical areas of the breeding range because the timing for peak oviposition and larval feeding likely differs by location. More information is needed about how monarchs find and use mature, naturally occurring milkweed plants. Understanding how females utilize these mature patches will allow researchers and managers to assess the worth of different milkweed species and the configuration of milkweed patches within habitat restoration sites.
As a whole, the results show that there are a few species that are most preferred for oviposition and would be best to use for restoration purposes (Table 1). Other considerations in choosing a species for restoration include matching the habitat preferences of species with the environmental conditions of the restoration site. Planting several milkweeds species with different habitat preferences may allow the persistence of milkweeds at a site despite variable weather conditions within and between years. Because larval survivorship is high on most species, with the exception of a couple (Table 1), planting a few species that are less preferred for oviposition will not compromise larval survival. See Table 1 for a summary of milkweed species' habitat requirements, ease of plug establishment, and utility for larvae and ovipositing females. We designate a species as recommended for restoration if plugs were easy to establish, had high larval survivorship, and medium to high oviposition use.
Author Contributions
These studies were part of the Ph.D. project of VP; the plant and monarch monitoring were done under the supervision of DD and JP. VP, DD, and JP are responsible for the experimental designs. KB, RH, StB, SuB, DD, and JP contributed to site selection, experimental designs, and growing all milkweed species. The manuscript was prepared by VP and critically revised by JP, DD, RH, StB, and SuB.
Funding
This work was partially funded by the USDA National Institute of Food and Agriculture, Hatch project number 1009926 (IOW05478), Prairie Biotics, Inc., The Center for Global and Regional Climate Research (CGRER), and by the USDA, Natural Resources Conservation Service's Conservation Innovation Grant program under Agreement Number 69-3A75-16-006. The Iowa Monarch Conservation Consortium provided additional support.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Mention of a proprietary product does not constitute an endorsement or a recommendation by Iowa State University or USDA for its use. The authors would like to thank Ali Ford, Nancy Shryock, Cory Haggard, Jacqueline Appelhans, Royce Bitzer, Kristen Siewert, Kirk Larsen, Linda Powell, Steve Sorensen, Lyle Rossiter, Dallas Maxwell, Randy Breach, Steve Jonas, Nick Howell, Logan Wallace, Myron Rees, Brandyn Chapman, Ken Pecinovsky, Matt Schnabel, Terry Tuttle, and Chris Beedle for their help with demonstration site establishment, plant replacement, and data collection. The authors would like to thank Nick Oppedal, Kelsey Fisher, Teresa Blader, and Niranjana Krishnan for their help planting, transplanting, and watering milkweed plants grown at Iowa State for this project, and Lincoln Brower for his comments which improved the clarity of this manuscript. We also thank Jay Diffendorfer and two reviewers for their constructive criticism which greatly improved this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2018.00169/full#supplementary-material
References
Baker, A. M., and Potter, D. A. (2018). Colonization and usage of eight milkweed (Asclepias) species by monarch butterflies and bees in urban garden settings. J. Insect Conserv. 1–14. doi: 10.1007/s10841-018-0069-5
Bartholomew, C., and Yeargan, M. (2002). Phenology of milkweed (Asclepiadaceae) growth and monarch (Lepidoptera: Nymphalidae) reproduction in Kentucky and ovipositional preference between common and honeyvine milkweed. J. Kans. Entomol. Soc. 74, 211–220.
Berdegué, M., Reitz, S. R., and Trumble, J. T. (1998). Host plant selection and development in Spodoptera exigua: do mother and offspring know best? Entomol. Exp. Appl. 89, 57–64. doi: 10.1046/j.1570-7458.1998.00381.x
Brower, L. P., Taylor, O. R., Williams, E. H., Slayback, D. A., Zubieta, R. R., and Ramirez, M. I. (2012). Decline of monarch butterflies overwintering in Mexico: is the migratory phenomenon at risk? Insect Conserv. Div. 5, 95–100. doi: 10.1111/j.1752-4598.2011.00142.x
Calvert, W. H. (1999). Patterns in the spatial and temporal use of Texas milkweeds (Asclepiadaceae) by the monarch butterfly (Danaus plexippus L.) during fall, 1996. J. Lepidopterists Soc. 53, 37–44.
Casagrande, R., and Dacey, J. (2007). Monarch butterfly oviposition on swallow-worts (Vincetoxicum spp.). Environ. Entomol. 36, 631–636. doi: 10.1603/0046-225X(2007)36[631:MBOOSV]2.0.CO;2
Ceballos, G., and Ehrlich, P. R. (2002). Mammal population losses and the extinction crisis. Science 296, 904–907. doi: 10.1126/science.1069349
Cohen, J. A., and Brower, L. P. (1982). Oviposition and larval success of wild monarch butterflies (Lepidoptera: Danaidae) in relation to host plant size and cardenolide concentration. J. Kans. Entomol. Soc. 55, 343–348.
DiTommaso, A., and Losey, J. E. (2003). Oviposition preference and larval performance of monarch butterflies (Danaus plexippus) on two invasive swallow-wort species. Entomol. Exp. Appl. 108, 205–209. doi: 10.1046/j.1570-7458.2003.00089.x
Eilers, L. J., Roosa, D. M. (1994). The Vascular Plants of Iowa: an Annotated Checklist and Natural History. Iowa, IA: University of Iowa Press.
Espeset, A. E., Harrison, J. G., Shapiro, A. M., Nice, C. C., Thorne, J. H., Waetjen, D. P., et al. (2016). Understanding a migratory species in a changing world: climatic effects and demographic declines in the western monarch revealed by four decades of intensive monitoring. Oecologia 181, 819–830. doi: 10.1007/s00442-016-3600-y
Flockhart, D. T., Pichancourt, J. B., Norris, D. R., and Martin, T. G. (2015). Unraveling the annual cycle in a migratory animal: breeding season habitat loss drives population declines of monarch butterflies. J. Anim. Ecol. 84, 155–165. doi: 10.1111/1365-2656.12253
Flockhart, D. T., Brower, L. P., Ramirez, M. I., Hobson, K. A., Wassenaar, L. I., Altizer, S., et al. (2017). Regional climate on the breeding grounds predicts variation in the natal origin of monarch butterflies overwintering in Mexico over 38 years. Glob. Change Biol. 23, 2565–2576. doi: 10.1111/gcb.13589
Gratton, C., and Welter, S. C. (1998). Oviposition preference and larval performance of Liriomyza helianthi (Diptera: Agromyzidae) on normal and novel host plants. Environ. Entomol. 27, 926–935. doi: 10.1093/ee/27.4.926
Haribal, M., and Renwick, J. A. (1996). Oviposition stimulants for the monarch butterfly: flavonol glycosides from Asclepias curassavica. Phytochemistry 41, 139–144. doi: 10.1023/A:1022377618562
Haribal, M., and Renwick, J. A. (1998a). Identification and distribution of oviposition stimulants for monarch butterflies in hosts and non-hosts. J. Chem. Ecol. 24, 891–904. doi: 10.1023/A:1022363329446
Haribal, M., and Renwick, J. A. (1998b). Differential postalightment oviposition behavior of monarch butterflies on Asclepias species. J. Insect Behav. 11, 507–538.
Hayden, A. (1919). Notes on the floristic features of a prairie province in central Iowa. Proc. Iowa Acad. Sci. 25:376.
Inamine, H., Ellner, S. P., Springer, J. P., and Agrawal, A. A. (2016). Linking the continental migratory cycle of the monarch butterfly to understand its population decline. Oikos 125, 1081–1091. doi: 10.1111/oik.03196
Kaul, R. B., Rolfsmeier, S. B., and Esch, J. J. (1991). The distribution and reproductive phenology of the milkweeds Asclepiadaceae: Asclepias and Cynanchum in Nebraska. Trans. Nebraska Acad. Sci. Affiliat. Soc. 18, 127–140.
Kerr, J. T., and Cihlar, J. (2004). Patterns and causes of species endangerment in Canada. Ecol. Appl. 14, 743–753. doi: 10.1890/02-5117
Ladner, D. T., and Altizer, S. M. (2005). Oviposition preference and larval performance of North American monarch butterflies on four Asclepias species. Entomol. Exp. Appl. 116, 9–20. doi: 10.1111/j.1570-7458.2005.00308.x
Landis, T. D. (2013). Monarchs (Danaus plexippus) and milkweeds (Asclepias species) the current situation and methods for propagating milkweeds. Native Plants J. 14, 5–16. doi: 10.3368/npj.14.1.5
Malcolm, S. B. (1991). “Cardenolide-mediated interactions between plants and herbivores,” in Herbivores: Their Interactions With Secondary Plant Metabolites, eds G. A. Rosenthal and M. R. Berenbaum (San Diego, CA: Academic Press, Inc.), 251–291.
Malcolm, S. B., Cockrell, B. J., and Brower, L. P. (1989). Cardenolide fingerprint of monarch butterflies reared on common milkweed, Asclepias syriaca L. J. Chem. Ecol. 15, 819–853. doi: 10.1007/BF01015180
Martin, A. R., and Burnside, O. C. (1980). Common milkweed: weed on the increase. Weeds Today Early 11, 19–20.
Mayhew, P. J. (1997). Adaptive patterns of host-plant selection by phytophagous insects. Oikos 79, 417–428. doi: 10.2307/3546884
Mayhew, P. J. (2001). Herbivore host choice and optimal bad motherhood. Trends Ecol. Evol. 16, 165–167. doi: 10.1016/S0169-5347(00)02099-1
Means, D. B., and Simberloff, D. (1987). The peninsula effect: habitat-correlated species decline in Florida's herpetofauna. J. Biogeogr. 14, 551–568. doi: 10.2307/2844880
Oberhauser, K. S., Wiederholt, R., Diffendorfer, J. E., Semmens, D., Ries, L., Thogmartin, W. E., et al. (2016). A trans-national monarch butterfly population model and implications for regional conservation priorities. Ecol. Entomol. 42, 51–60. doi: 10.1111/een.12351
Pimm, S. L., and Raven, P. (2000). Extinction by numbers. Nature 403, 843–845. doi: 10.1038/35002708
Pleasants, J. M. (2015). “Monarch butterflies and agriculture,” in Monarchs in a Changing World: Biology and Conservation of an Iconic Butterfly, eds K. S. Oberhauser, S. Altizer, and K. Nail (Ithaca, NY: Cornell University Press), 169–178.
Pleasants, J. M., and Oberhauser, K. S. (2013). Milkweed loss in agricultural fields because of herbicide use: effect on the monarch butterfly population. Insect Conserv. Div. 6, 135–144. doi: 10.1111/j.1752-4598.2012.00196.x
Pleasants, J. M. (2017). Milkweed restoration in the Midwest for Monarch butterfly recovery: estimates of milkweeds lost, milkweeds remaining and milkweeds that must be added to increase the Monarch population. Insect Conserv. Div. 10, 42–53. doi: 10.1111/icad.12198
Pocius, V. M., Debinski, D. M., Bidne, K. G., Hellmich, R. L., and Hunter, F. K. (2017a). Performance of early instar monarch butterflies (Danaus plexippus L.) on nine milkweed species native to Iowa. J. Lepidopterist's Soc. 71, 153–161. doi: 10.18473/lepi.71i3.a5
Pocius, V. M., Debinski, D. M., Pleasants, J. M., Bidne, K. G., and Hellmich, R. L. (2018). Monarch butterflies do not place all of their eggs in one basket: oviposition on nine Midwestern milkweed species. Ecosphere 9:e02064. doi: 10.1002/ecs2.2064
Pocius, V. M., Debinski, D. M., Pleasants, J. M., Bidne, K. G., Hellmich, R. L., and Brower, L. P. (2017b). Milkweed matters: Monarch butterfly (Lepidoptera: Nymphalidae Danaus plexippus) survival and development on nine Midwestern milkweed species. Environ. Entomol. 46, 1098–1105. doi: 10.1093/ee/nvx137
Rasmann, S., and Agrawal, A. A. (2011). Latitudinal patterns in plant defense: evolution of cardenolides, their toxicity and induction following herbivory. Ecol. Lett. 14, 476–483. doi: 10.1111/j.1461-0248.2011.01609.x
Robertson, G. F., Zalucki, M. P., and Paine, T. D. (2015). Larval host choice of the monarch butterfly (Danaus plexxippus) on four native California desert milkweed species. J. Insect Behav. 28, 582–592. doi: 10.1007/s10905-015-9524-2
Roeske, C. N., Seiber, J. N., Brower, L. P., and Moffitt, C. M. (1976). “Milkweed cardenolides and their comparative processing by monarch butterflies (Danaus plexxippus L.)” in Biochemical Interaction Between Plants and Insects. Vol. 10, ed J. W. Wallace and R. L. Mansell (New York, NY: Springer US) 93–167.
Schultz, C. B., Brown, L. M., Pelton, E., and Crone, E. E. (2017). Citizen science monitoring demonstrates dramatic declines of monarch butterflies in western North America. Biol. Conserv.Short Commun. 214, 343–446. doi: 10.1016/j.biocon.2017.08.019
Team (2014). R: A Language and Environment for Statistical Computing. Vienna: Foundation for Statistical Computing.
Thogmartin, W. E., López-Hoffman, L., Rohweder, J., Diffendorfer, J., Drum, R., Semmens, D., et al. (2017). Restoring monarch butterfly habitat in the Midwestern US: ‘all hands on deck'. Environ. Res. Lett. 12:074005. doi: 10.1088/1748-9326/aa,7637
Venter, O., Brodeur, N. N., Nemiroff, L., Belland, B., Dolinsek, I. J., and Grant, J. W. (2006). Threats to endangered species in Canada. Bioscience 56, 903–910. doi: 10.1641/0006-3568(2006)56[903:TTESIC]2.0.CO;2
Wassenaar, L. I., and Hobson, K. A. (1998). Natal origins of migratory monarch butterflies at wintering colonies in Mexico: new isotopic evidence. Proc. Nat. Acad. Sci. U.S.A. 95, 15436–15439. doi: 10.1073/pnas.95.26.15436
Wilcove, D. S., Rothstein, D., Dubow, J., Phillips, A., and Losos, E. (1998). Quantifying threats to imperiled species in the United States. Bioscience 45, 607–615. doi: 10.2307/1313420
Woodson, R. E. Jr. (1954). The North American species of Asclepias L. Ann. Mo. Bot. Gard. 41, 1–211. doi: 10.2307/2394652
Xu, X., Xie, Y., Qi, K., Luo, Z., and Wang, X. (2018). Detecting the response of bird communities and biodiversity to habitat loss and fragmentation due to urbanization. Sci. Tot. Environ. 624, 1561–1576. doi: 10.1016/j.scitotenv.2017.12.143
Yeargan, K. V., and Allard, C. M. (2005). Comparison of common milkweed and honeyvine milkweed (Asclepiadaceae) as host plants for monarch larvae (Lepidoptera: Nymphalidae). J. Kans. Entomol. Soc. 78, 247–251. doi: 10.2317/0407.40.1
Zalucki, M., and Kitching, R. (1982). Dynamics of oviposition in Danaus plexippus (Insecta: Lepidoptera) on milkweed, Asclepias spp. J. Zool. 198, 103–116. doi: 10.1111/j.1469-7998.1982.tb02063.x
Zalucki, M. P., and Brower, L. P. (1992). Survival of first instar larvae of Danaus plexippus (Lepidoptera: Danainae) in relation to cardiac glycoside and latex content of Asclepias humistrata (Asclepiadaceae). Chemoecology 3, 81–93. doi: 10.1007/BF01245886
Zalucki, M. P., Brower, L. P., and Malcolm, S. B. (1990). Oviposition by Danaus plexippus in relation to cardenolide content of three Asclepias species in the southeastern USA. Ecol. Entomol. 15, 231–240. doi: 10.1111/j.1365-2311.1990.tb00804.x
Zaya, D. N., Pearse, I. S., and Spyreas, G. (2017). Long-term trends in midwestern milkweed abundances and their relevance to monarch butterfly declines. Bioscience 67, 343–356. doi: 10.1093/biosci/biw186
Keywords: Danaus plexippus, milkweed species (Asclepias spp), oviposition preference, habitat restoration, conservation
Citation: Pocius VM, Pleasants JM, Debinski DM, Bidne KG, Hellmich RL, Bradbury SP and Blodgett SL (2018) Monarch Butterflies Show Differential Utilization of Nine Midwestern Milkweed Species. Front. Ecol. Evol. 6:169. doi: 10.3389/fevo.2018.00169
Received: 22 June 2018; Accepted: 04 October 2018;
Published: 25 October 2018.
Edited by:
Jay E. Diffendorfer, United States Geological Survey, United StatesReviewed by:
Jennifer Lesley Silcock, The University of Queensland, AustraliaPanagiotis Milonas, Benaki Phytopathological Institute, Greece
Copyright © 2018 Pocius, Pleasants, Debinski, Bidne, Hellmich, Bradbury and Blodgett. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Victoria Marie Pocius, vmpocius@gmail.com
 Victoria Marie Pocius
Victoria Marie Pocius John M. Pleasants
John M. Pleasants Diane M. Debinski
Diane M. Debinski Keith G. Bidne4
Keith G. Bidne4  Richard L. Hellmich
Richard L. Hellmich