Abstract
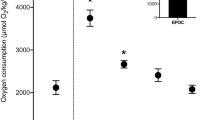
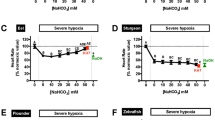
Elevated plasma potassium levels (hyperkalemia), reduced plasma pH (acidosis), reduced blood oxygen content, and elevated temperatures are associated with species-specific rates of at-vessel and post-release mortality in elasmobranch fishes. The mechanism linking these physiological disturbances to mortality remains undetermined however, and we hypothesize that the proximate cause is reduced myocardial function. We measured changes in the functional properties of isolated ventricular myocardial strips from clearnose skate (Rostroraja eglanteria), smooth dogfish (Mustelus canis), and sandbar shark (Carcharhinus plumbeus) when subjected to the following stressors (both in isolation and in combination): hyperkalemia (7.4 mM K+), acidosis (from 7.9 to 7.1), and reduced oxygen (to 31% O2 saturation) applied at temperatures 5 °C above and below holding temperatures. We selected these species based on phylogenetic distance, diverse routine activity levels, and their tolerance to capture and transport. Stressors had a few significant species-specific detrimental impacts on myocardial function (e.g., a 33–45% decrease in net force under acidosis + low O2). Net force production of myocardial strips from clearnose skate and smooth dogfish approximately doubled following exposure to isoproterenol, demonstrating that these species possess beta-adrenergic receptors and that their stimulation could provide a mechanism for preservation of cardiac function during stress. Our results suggest that disruption of physiological homeostasis associated with capture may fatally impair cardiac function in some elasmobranch species, although research with more severe stressors is needed.





Similar content being viewed by others
Data availability
Data is freely available through the William and Mary data repository at https://doi.org/10.25773/aamf-fb65
Code availability
Not applicable.
References
Altimiras J, Larsen E (2000) Non-invasive recording of heart rate and ventilation rate in rainbow trout during rest and swimming. Fish go wireless! J Fish Biol 57:197–209
Arlinghaus R et al (2007) Understanding the complexity of catch-and-release in recreational fishing: an integrative synthesis of global knowledge from historical, ethical, social, and biological perspectives. Rev Fish Sci 15:75–167
Breitburg D et al (2018) Declining oxygen in the global ocean and coastal waters. Science. https://doi.org/10.1126/science.aam7240
Brill R, Bushnell P, Schroff S, Seifert R, Galvin M (2008) Effects of anaerobic exercise accompanying catch-and-release fishing on blood-oxygen affinity of the sandbar shark (Carcharhinus plumbeus, Nardo). J Exp Mar Biol Ecol 354:132–143
Brill RW, Bushnell PG (2001) The cardiovascular system of tunas. In: Block BA, Stevens ED (eds) Fish physiology, vol 19. Academic Press, San Diego, pp 79–120
Brill RW, Lai NC (2016) Elasmobranch cardiovascular system. In: Shadwick RE, Farrell AP, Brauner CJ (eds) Physiology of elasmobranch fishes: internal processes. Fish physiology, vol 34. Academic Press, San Diego
Bushnell PG, Lutz PL, Steffensen JF, Oikari A, Gruber SH (1982) Increases in arterial blood oxygen during exercise in the lemon shark (Negaprion brevirostris). J Compar Physiol B 147:41–47. https://doi.org/10.1007/bf00689288
Butler PJ, Taylor EW (1975) The effect of progressive hypoxia on respiration in the dogfish (Scyliorhinus canicula) at different seasonal temperatures. J Exp Biol 63:117–130
Campana SE, Joyce W, Manning MJ (2009) Bycatch and discard mortality in commercially caught blue sharks Prionace glauca assessed using archival satellite pop-up tags. Mar Ecol Prog Ser 387:241–253
Cann M (2004) Bicarbonate stimulated adenylyl cyclases. IUBMB Life 56:529–534. https://doi.org/10.1080/15216540400013861
Carlson JK (1999) Occurrence of neonate and juvenile sandbar sharks, Carcharhinus plumbeus, in the northeastern Gulf of Mexico. Fish Bull 97
Carlson JK, Goldman KJ, Lowe CG (2004) Metabolism, energetic demand, and endothermy. In: Carrier JC, Musick JA, Heithaus MR (eds) Biology of sharks and their relatives, Marine biology, vol 10. CRC Press, Boca Raton, pp 203–224
Clark TD, Ryan T, Ingram B, Woakes A, Butler P, Frappell PB (2005) Factorial aerobic scope is independent of temperature and primarily modulated by heart rate in exercising Murray cod (Maccullochella peelii peelii). Physiol Biochem Zool 78:347–355
Cliff G, Thurman GD (1984) Pathological and physiological effects of stress during capture and transport in the juvenile dusky shark, Carcharhinus obscurus. Comp Biochem Physiol Part A 78:167–173
Cox GK, Brill RW, Bonaro KA, Farrell AP (2017) Determinants of coronary blood flow in sandbar sharks, Carcharhinus plumbeus. J Comp Physiol B 187:315–327. https://doi.org/10.1007/s00360-016-1033-x
Cox GK, Kennedy GE, Farrell AP (2016) Morphological arrangement of the coronary vasculature in a shark (Squalus sucklei) and a teleost (Oncorhynchus mykiss). J Morphol 277:896–905
Danylchuk AJ, Suski CD, Mandelman JW, Murchie KJ, Haak CR, Brooks AM, Cooke SJ (2014) Hooking injury, physiological status and short-term mortality of juvenile lemon sharks (Negaprion bevirostris) following catch-and-release recreational angling. Conserv Physiol 2:cot036. https://doi.org/10.1093/conphys/cot036
Dapp DR, Huveneers C, Walker TI, Drew M, Reina RD (2016) Moving from measuring to predicting bycatch mortality: predicting the capture condition of a longline-caught pelagic shark. Front Marine Sci. https://doi.org/10.3389/fmars.2015.00126
Dowd WW, Brill RW, Bushnell PG, Musick JA (2006) Standard and routine metabolic rates of juvenile sandbar sharks (Carcharhinus plumbeus) including the effects of body mass and acute temperature change. Fish Bull 104:323–331
Driedzic WR, Gesser H (1988) Differences in force-frequency relationships and calcium dependency between elasmobranch and teleost hearts. J Exp Biol 140:227–241
Dulvy NK et al (2014) Extinction risk and conservation of the world’s sharks and rays. eLife 2014:e00590
Dziergwa J, Singh S, Bridges CR, Kerwath SE, Enax J, Auerswald L (2019) Acid-base adjustments and first evidence of denticle corrosion caused by ocean acidification conditions in a demersal shark species. Sci Rep 9:18668. https://doi.org/10.1038/s41598-019-54795-7
El-Sayed MF, Gesser H (1989) Sarcoplasmic reticulum, potassium, and cardiac force in rainbow trout and plaice. Am J Physiol 257:R599–R604
Eliason EJ, Anttila K (2017) Temperature and the cardiovascular system. In: Fish Physiology, vol 36. Elsevier, Amsterdam, pp 235–297
Emery SH (1985) Hematology and cardiac morphology in the great white shark, Carcharodon carcharias. Mem South Calif Acad Sci 9:73–80
Erickson DL, Berkeley SA (2008) Methods to reduce bycatch mortality in longline fisheries Sharks of the open ocean: biology, fisheries and conservation Edited by MD Camhi, EK Pikitch, and EA Babcock Blackwell Publishing, Oxford, UK:462–471
Fange R, Ostlund E (1954) The effects of adrenaline, noradrenaline, tyramine, and other drugs on the isolated heart from marine vertebrates and and a cephalopod (Eledone cirrosa). Acta Zool (Stockh):289–305
Farrell A, Farrell N, Jourdan H, Cox G (2012) A perspective on the evolution of the coronary circulation in fishes and the transition to terrestrial life. In: Ontogeny and Phylogeny of the Vertebrate Heart. Springer, pp 75–102
Farrell A, Jones D (1992) The heart. In: Hoar W, Randall DJ, Farrell AP (eds) Fish Physiology, vol 12A. Academic Press, San Diego
Farrell A, MacLeod K, Driedzic W, Wood S (1983) Cardiac performance in the in situ perfused fish heart during extracellular acidosis: interactive effects of adrenaline. J Exp Biol 107:415–429
Farrell A, Milligan C (1986) Myocardial intracellular pH in a perfused rainbow trout heart during extracellular acidosis in the presence and absence of adrenaline. J Exp Biol 125:347–359
Farrell AP (1991) From hagfish to tuna: a perspective on cardiac function in fish. Physiol Zool 64:1137–1164
Frick LH, Reina RD, Walker TI (2010) Stress related physiological changes and post-release survival of Port Jackson sharks (Heterodontus portusjacksoni) and gummy sharks (Mustelus antarcticus) following gill-net and longline capture in captivity. J Exp Mar Biol Ecol:29–37
Gallagher AJ, Serafy JE, Cooke SJ, Hammerschlag N (2014) Physiological stress response, reflex impairment, and survival of five sympatric shark species following experimental capture and release. Mar Ecol Prog Ser 496:207–218
Gamperl AK, Shiels HA (2014) Cardiovascular system. In: Evans DH, Claiborne JB, Currie S (eds) The physiology of fishes, 4th edn. Taylor & Francis Inc, Bosa Roca, pp 33–79
Gesser H, Jorgensen E (1982) pHi, contractility and Ca-balance under hypercapnic acidosis in the myocardium of different vertebrate species. J Exp Biol 96:405–412
Gesser H, Poupa O (1979) Effects of different types of acidosis and Ca2+ on cardiac contractility in the flounder (Pleuronectes flesus). J Comp Physiol A 131:293–296
Gesser H, Rodnick KJ (2019) Is the teleost heart oxygen limited? – Insights using “hyperoxic” incubations of contracting cardiac tissue from rainbow trout. Comp Biochem Physiol Part A. https://doi.org/10.1016/j.cbpa.2019.01.027
Gillis TE, Marshall CR, Xue XH, Borgford TJ, Tibbits GF (2000) Ca2+ binding to cardiac troponin C: effects of temperature and pH on mammalian and salmonid isoforms. Am J Physiol Regulat Integrat Comp Physiol 279:R1707–R1715
Hanson D, Johansen K (1970) Relationship of gill ventilation and perfusion in pacific dogfish, Squalus suckleyi. J Fish Res Board Can 27:551–564
Hanson LM, Farrell AP (2007) The hypoxic threshold for maximum cardiac performance in rainbow troutOncorhynchus mykiss(Walbaum) during simulated exercise conditions at 18° C. J Fish Biol 71:926–932. https://doi.org/10.1111/j.1095-8649.2007.01533.x
Hanson LM, Obradovich S, Mouniargi J, Farrell AP (2006) The role of adrenergic stimulation in maintaining maximum cardiac performance in rainbow trout (Oncorhynchus mykiss) during hypoxia, hyperkalemia and acidosis at 10 °C. J Exp Biol 209:2442–2451. https://doi.org/10.1242/jeb.02237
Hiatt EP (1943) The action of adrenaline, acetylcholine and potassium in relation to the innervation of the isolated auricle of the spiny dogfish (Squalus acanthias). Am J Physiol 139:45–48
Hickey AJ, Renshaw GM, Speers-Roesch B, Richards JG, Wang Y, Farrell AP, Brauner CJ (2012) A radical approach to beating hypoxia: depressed free radical release from heart fibres of the hypoxia-tolerant epaulette shark (Hemiscyllum ocellatum). J Comp Physiol B 182:91–100. https://doi.org/10.1007/s00360-011-0599-6
Hight BV et al (2007) Plasma catecholamine levels as indicators of the post-release survivorship of juvenile pelagic sharks caught on experimental drift longlines in the Southern California Bight. Mar Freshw Res 58:145. https://doi.org/10.1071/mf05260
Hoolihan JP et al (2011) Evaluating post-release behavior modification in large pelagic fish deployed with pop-up satellite archival tags. ICES J Mar Sci 68:880–889
Horodysky AZ, Cooke SJ, Graves JE, Brill RW (2016) Fisheries conservation on the high seas: linking conservation physiology and fisheries ecology for the management of large pelagic fishes. Conserv Physiol 4:cov059. https://doi.org/10.1093/conphys/cov059
Hyatt MW, Anderson PA, O’Donnell PM, Berzins IK (2011) Assessment of acid-base derangements among bonnethead (Sphyrna tiburo), bull (Carcharhinus leucas), and lemon (Negaprion brevirostris) sharks from gillnet and longline capture and handling methods. Comp Biochem Physiol A 162:113–120
Iversen NK, Dupont-Prinet A, Findorf I, McKenzie DJ, Wang T (2010) Autonomic regulation of the heart during digestion and aerobic swimming in the European sea bass (Dicentrarchus labrax). Comp Biochem Physiol Part A 156:463–468
Joyce W, Ozolina K, Mauduit F, Ollivier H, Claireaux G, Shiels HA (2016) Individual variation in whole-animal hypoxia tolerance is associated with cardiac hypoxia tolerance in a marine teleost. Biol Lett 12:20150708. https://doi.org/10.1098/rsbl.2015.0708
Kalinin A, Gesser H (2002) Oxygen consumption and force development in turtle and trout cardiac muscle during acidosis and high extracellular potassium. J Comp Physiol B 172:145–151. https://doi.org/10.1007/s00360-001-0237-9
Kieffer JD (2000) Limits to exhaustive exercise in fish. Comp Biochem Physiol Part A 126:161–179
Kneebone J, Chisholm J, Bernal D, Skomal G (2013) The physiological effects of capture stress, recovery, and post-release survivorship of juvenile sand tigers (Carcharinas taurus) caught on rod and reel. Fish Res 147:103–114
Lai N, Graham IB, Burnett L (1990) Blood respiratory properties and the effect of swimming on blood gas transport in the leopard shark Triakis semifasciata. J Exp Biol 151:161–173
Lai NC, Korsmeyer KE, Katz S, Holts DB, Laughlin LM, Graham JB (1997) Hemodynamics and blood properties of the shortfin mako shark (Isurus oxyrinchus). Copeia 1997:424–428
Larsen J, Bushnell P, Steffensen J, Pedersen M, Qvortrup K, Brill R (2017) Characterization of the functional and anatomical differences in the atrial and ventricular myocardium from three species of elasmobranch fishes: smooth dogfish (Mustelus canis), sandbar shark (Carcharhinus plumbeus), and clearnose skate (Raja eglanteria). J Comp Physiol B 187:291–313. https://doi.org/10.1007/s00360-016-1034-9
Lowe T, Wells R, Baldwin J (1995) Absence of regulated blood-oxygen transport in response to strenuous exercise by the shovelnosed ray, Rhinobatos typus. Mar Freshw Res 46:441–446
Luer CA, Gilbert PW (1985) Mating behavior, egg deposition, incubation period, and hatching in the clearnose skate, Raja eglanteria. Environ Biol Fishes 13:161–171
Luer CA, Walsh CJ, Bodine AB, Wyffels JT (2007) Normal embryonic development in the clearnose skate, Raja eglanteria, with experimental observations on artificial insemination. Environ Biol Fishes 80:239–255. https://doi.org/10.1007/s10641-007-9219-4
Mandelman J, Skomal G (2009) Differential sensitivity to capture stress assessed by blood acidñbase status in five carcharhinid sharks. J Comp Physiol B 179:267–277
Mandelman JW, Farrington MA (2007) The physiological status and mortality associated with otter-trawl capture, transport, and captivity of an exploited elasmobranch Squalus acanthias. ICES J Mar Sci 64:122–130
Manire C, Hueter R, Hull E, Spieler R (2001) Serological changes associated with gill-net capture and restraint in three species of sharks. Trans Am Fish Soc 130:1038–1048
Marshall H (2015) Investigations in the stress physiology and survival of elasmobranch fishes, with applications to fisheries management., University of Massachusetts Dartmouth
Marshall H, Field L, Afiadata A, Sepulveda C, Skomal G, Bernal D (2012) Hematological indicators of stress in longline-captured sharks. Comp Biochem Physiol A 162:121–129
McKenzie DJ et al (2016) Conservation physiology of marine fishes: state of the art and prospects for policy. Conserv Physiol 4:cow046. https://doi.org/10.1093/conphys/cow046
Moyes CD, Fragoso N, Musyl MK, Brill RW (2006) Predicting postrelease survival in large pelagic fish. Trans Am Fish Soc 135:1389–1397
Musyl MK et al (2011) Postrelease survival, vertical and horizontal movements, and thermal habitats of five species of pelagic sharks in the central. Pacific Ocean Fish Bull 109:341–368
Musyl MK, Gilman EL (2019) Meta-analysis of post-release fishing mortality in apex predatory pelagic sharks and white marlin. Fish Fish. https://doi.org/10.1111/faf.12358
Musyl MK, Moyes CD, Brill RW, Fragoso NM (2009) Factors influencing mortality estimates in post-release survival studies. Mar Ecol Progress 396:157–159
Nielsen JS, Gesser H (2001) Effects of high extracellular [K+] and adrenaline on force development, relaxation and membrane potential in cardiac muscle from freshwater turtle and rainbow trout. J Exp Biol 204:261–268
Nilsson S (1983) Autonomic nerve function in the vertebrates. Springer-Verlag, Heidleberg
Paajanen V, Vornanen M (2003) Effects of Chronic Hypoxia on Inward Rectifier K+ Current (IK1) in Ventricular Myocytes of Crucian Carp (Carassius carassius). Heart J Membr Biol 194:119–127. https://doi.org/10.1007/s00232-003-2032-x
Pinheiro J, Bates D, DebRoy S, Sarkar D, Team RC (2014) nlme: Linear and Nonlinear Mixed Effects Models R package version 31–117
Randall DJ (1982) The control of respiration and circulation in fish during exercise and hypoxia. J Exp Biol 100:275–288
Rodnick KJ, Gamperl AK, Nash GW, Syme DA (2014) Temperature and sex dependent effects on cardiac mitochondrial metabolism in Atlantic cod (Gadus morhua L.). J Therm Biol 44:110–118. https://doi.org/10.1016/j.jtherbio.2014.02.012
Sandblom E, Axelsson M, Farrell AP (2006) Central venous pressure and mean circulatory filling pressure in the dogfish Squalus acanthias: adrenergic control and role of the pericardium. Am J Physiol Regul Integr Comp Physiol 291:R1465-1473. https://doi.org/10.1152/ajpregu.00282.2006
Sandblom E, Cox GK, Perry SF, Farrell AP (2009) The role of venous capacitance, circulating catecholamines, and heart rate in the hemodynamic response to increased temperature and hypoxia in the dogfish. Am J Physiol Regul Integr Comp Physiol 296:R1547-1556. https://doi.org/10.1152/ajpregu.90961.2008
Scharold J, Gruber SH (1991) Telemetered heart rate as a measure of metabolic rate in the lemon shark, Negaprion brevirostris. Copeia 1991:942–953
Scharold J, Lai NC, Lowell WR, Graham JB (1989) Metabolic rate, heart rate, and tailbeat frequency during sustained swimming in the leopard shark Triakis semifasciata. Exp Biol 48:223–230
Schlenker LS, Latour RJ, Brill RW, Graves JE (2016) Physiological stress and post-release mortality of white marlin (Kajikia albida) caught in the United States recreational fishery. Conserv Physiol 4:cov066. https://doi.org/10.1093/conphys/cov066
Schwieterman GD, Crear DP, Anderson BN, Lavoie DR, Sulikowski JA, Bushnell PG, Brill RW (2019) Combined Effects of Acute Temperature Change and Elevated pCO2 on the Metabolic Rates and Hypoxia Tolerances of Clearnose Skate (Rostaraja eglanteria), Summer Flounder (Paralichthys dentatus), and Thorny Skate (Amblyraja radiata). Biology (Basel). https://doi.org/10.3390/biology8030056
Shiels HA, Farrell AP (1997) The effect of temperature and adrenaline on the relative importance of the sarcoplasmic reticulum in contributing Ca2+ to force development in isolated ventricular trabeculae from rainbow trout. J Exp Biol 200:1607–1621
Shiels HA, Galli GL (2014) The sarcoplasmic reticulum and the evolution of the vertebrate heart. Physiology (Bethesda) 29:456–469. https://doi.org/10.1152/physiol.00015.2014
Shiels HA, Santiago DA, Galli GL (2010) Hypercapnic acidosis reduces contractile function in the ventricle of the armored catfish Pterygoplichthys pardalis. Physiol Biochem Zool 83:366–375. https://doi.org/10.1086/644759
Shiels HA, Vornanen M, Farrell AP (2002) The force-frequency relationship in fish hearts—a review. Comp Biochem Physiol Part A 132:811–826
Skomal GB (2006) The physiological effects of capture stress on post-release survivorship of sharks, tunas, and marlin. Boston University, Boston
Skomal GB (2007) Evaluating the physiological and physical consequences of capture on post-release survivorship in large pelagic fishes. Fish Manage Ecol 14:81–89
Skomal GB, Bernal D (2010) Physiological responses to stress in sharks. In: Carrier JC, Musick JA, Heithaus MR (eds) Sharks and their relatives: biodiversity, adaptive physiology and conservation. CRC Press, Boca Raton
Speers-Roesch B, Brauner CJ, Farrell AP, Hickey AJ, Renshaw GM, Wang YS, Richards JG (2012) Hypoxia tolerance in elasmobranchs. II. Cardiovascular function and tissue metabolic responses during progressive and relative hypoxia exposures. J Exp Biol 215:103–114. https://doi.org/10.1242/jeb.059667
Stevens JD, Bonfil R, Dulvy NK, Walker PA (2000) The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems. ICES J Mar Sci 57:476–494
Thompson AP, O’Shea JE (1997) The unusual adrenergic-like excitatory action of acetylcholine on the ventricular cardiac muscle of the horned shark Heterodontus portusjacksoni. Physiol Zool 70:135–142
Tota B (1989) Myoarchitecture and vascularization of the elasmobranch heart ventricle. J Exp Zool 252:122–135
Tota B, Angelone T, Mancardi D, Cerra MC (2011) Hypoxia and anoxia tolerance of vertebrate hearts: an evolutionary perspective. Antioxid Redox Signal 14:851–862. https://doi.org/10.1089/ars.2010.3310
Tota B, Gattuso A (1996) Heart ventricle pumps in teleosts and elasmobranchs: a morphodynamic approach. J Exp Zool 275:162–171
Van Vliet BN, Metcalfe JD, Butler PJ, West NH (1988) The concentration dependence of the stimulatory effects of catecholamines on the rate and force of contraction of the heart of dogfish (Scyliorhinus canicula). J Exp Biol 140:549–555
Vornanen M (2016) The temperature dependence of electrical excitability in fish hearts. J Exp Biol 219:1941–1952. https://doi.org/10.1242/jeb.128439
Wells RMG, McIntyre RH, Morgan AK, Davie PS (1986) Physiological stress responses in big gamefish after capture observations on plasma chemistry and blood factors. Comp Biochem Physiol Part A 84A:565–571
Wendelaar-Bonga SE (1997) The stress response in fish. Physiol Rev 77:591–625
Whitney NM, White CF, Anderson PA, Hueter RE, Skomal GB (2017) The physiological stress response, postrelease behavior, and mortality of blacktip sharks (Carcharhinus limbatus) caught on circle and J-hooks in the Florida recreational fishery. Fish Bull 115:532–544
Wilson CM, Cox GK, Farrell AP (2015) The beat goes on: Cardiac pacemaking in extreme conditions. Comp Biochem Physiol A Mol Integr Physiol 186:52–60. https://doi.org/10.1016/j.cbpa.2014.08.014
Wood CM (1991) Acid-base and ion balance, metabolism, and their interactions, after exhaustive exercise in fish. J Exp Biol 160:285
Wosnick N, Navas CA, Niella YV, Monteiro-Filho ELA, Freire CA, Hammerschlag N (2018) Thermal imaging reveals changes in body surface temperatures of blacktip sharks (Carcharhinus limbatus) during air exposure. Physiol Biochem Zool 91:1005–1012. https://doi.org/10.1086/699484
Acknowledgements
We would like to thank two anonymous reviewers for their constructive comments on an earlier version of this article, T. Deemer for assistance in obtaining smooth dogfish, and D. Crear, G. Fay, and M. Winton for their assistance with statistical analysis. We would also like to thank D. Lavoie, R. Steffan, A. Sergio, T. Bigelow and the entire staff at the Virginia Institute of Marine Science Eastern Shore Laboratory for their unwavering logistical support and assistance in collecting specimens. Species silhouettes in Figs. 1-4 were provided by O. Shipley.
Funding
Support for G.S. was provided by National Science Foundation under Grant DGE‐1444317. Support for M.W. and D.B was provided by National Science Foundation under Grant IOS-1354593.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by GS and MW. The first draft of the manuscript was written by GS and MW and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All animal capture, care, and experimental protocols and procedures were approved by the William and Mary Institutional Animal Care and Use Committee (protocol: IACUC-2016-02-18-10947-rwbril).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Communicated by B. Pelster.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplemental Figure 1.
The functional parameters of ventricular myocardial strips isolated from clearnose skate (a), smooth dogfish (b), and sandbar shark (c) during exposure to control conditions and hyperkalemia (7.4 mM [K+]) at two temperatures. Each line connects the same strip under exposure to the control and hyperkalemia treatments. Unpaired data points are shown to demonstrate the range of values in the raw data, but were excluded from data analysis calculated fractional changes. (TIF 49677 KB)
Supplemental Figure 2.
The functional parameters of ventricular myocardial strips isolated from clearnose skate (a), smooth dogfish (b), and sandbar shark (c) during exposure to control conditions and acidosis + low O2 at two temperatures. Each line connects the same strip under exposure to the control and hyperkalemia treatments. Unpaired data points are shown to demonstrate the range of values in the raw data, but were excluded from data analysis calculated fractional changes (TIF 23707 KB)
Supplemental Figure 3.
The functional parameters of ventricular myocardial strips isolated from clearnose skate (a), smooth dogfish (b), and sandbar shark (c) during exposure to control conditions and combined stressors (hyperkalemia, acidosis, and low O2) at two temperatures. Each line connects the same strip under exposure to the control and hyperkalemia treatments. Unpaired data points are shown to demonstrate the range of values in the raw data but were excluded from data analysis calculated fractional changes (TIF 23538 KB)
Supplemental Figure 4.
The functional parameters ventricular myocardial strips isolated from clearnose skate (a), smooth dogfish (b), and sandbar shark (c) during exposure to control conditions and isoproterenol alone (“Control + Iso) at two temperatures. Each line connects the same strip under exposure to the control and hyperkalemia treatments. Unpaired data points are shown to demonstrate the range of values in the raw data, but were excluded from data analysis calculated fractional changes (TIF 23539 KB)
Supplemental Figure 5.
The functional parameters ventricular myocardial strips isolated from clearnose skate (a), smooth dogfish (b), and sandbar shark (c) during exposure to control conditions, and to hyperkalemia, acidosis, low O2, and isoproterenol (“Combined + Iso”) at two temperatures. Each line connects the same strip under exposure to the control and hyperkalemia treatments. Unpaired data points are shown to demonstrate the range of values in the raw data, but were excluded from data analysis calculated fractional changes (TIF 23442 KB)
Rights and permissions
About this article
Cite this article
Schwieterman, G.D., Winchester, M.M., Shiels, H.A. et al. The effects of elevated potassium, acidosis, reduced oxygen levels, and temperature on the functional properties of isolated myocardium from three elasmobranch fishes: clearnose skate (Rostroraja eglanteria), smooth dogfish (Mustelus canis), and sandbar shark (Carcharhinus plumbeus). J Comp Physiol B 191, 127–141 (2021). https://doi.org/10.1007/s00360-020-01328-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-020-01328-8