As Star Trek’s Spock once observed: “As a matter of cosmic history, it has always been easier to destroy than to create.”
The same is true inside human cells, explaining why Emory researchers’ recent accomplishment – finding a small-molecule compound that corrects a defective protein-protein interaction – is so significant for cancer research. It’s like putting Humpty Dumpty back together again.
Xiulei Mo, Haian Fu and colleagues have identified what they call a “mutation-directed molecular glue”. The glue restores a regulatory circuit that when defective, is responsible for acceleration of colorectal and pancreatic cancer. The results are reported in Cell Chemical Biology.

“It is very exciting, because this is a clear example of a protein-protein interaction stabilizer that can reactivate the lost function and reestablish tumor-suppressive activity,” says Fu, who is chair of Emory’s Pharmacology and Chemical Biology department and leader of Winship Cancer Institute’s Discovery & Developmental Therapeutics program.
Scientists are very good at finding inhibitors for enzymes that are overactive. But they have meager results as far as strengthening interactions that are weak or absent. There are existing examples of drugs that stabilize protein-protein interactions (transplant drugs rapamycin and cyclosporine), but they inhibit the function of the proteins they target, as intended.
An entire class of cancer-related genes is known as tumor suppressors, because they are guardians of the cell, slow down cell division, repair DNA mistakes, or tell cells to die when they are damaged. If the function of a tumor suppressor gene is impaired by mutation or deletion, then a cell is more likely to become cancerous.
“This work opens the door for similar studies on other tumor suppressors,” Fu says. “We hope that this unbiased chemical biology approach will work for both loss of interactions and decreased interactions.”
In the new paper, Mo and Fu decided to screen for compounds that could restore a protein-protein interaction that is weakened by a mutation in the gene SMAD4. The SMAD4 gene is frequently deleted or inactivated in colon and pancreatic cancers, disrupting cells’ ability to respond to growth inhibition by the cytokine TGF-beta. The R361H mutation is one of the most common in SMAD4. It does not completely abolish the interaction of the SMAD4 protein with its partner SMAD3, but weakens it.
Claire (Cong) Tang, co-first author of the paper, along with Mo developed a lab assay that depends on energy transfer between two fluorescent tags on the interacting proteins — one is on mutated SMAD4 and one is on SMAD3. When the two fluorescent tags are close enough together, then a color shift is observed. They used the resources of the Emory Chemical Biology Discovery Center to screen a library of bioactive compounds, and identified a couple that brought the two proteins back together again.
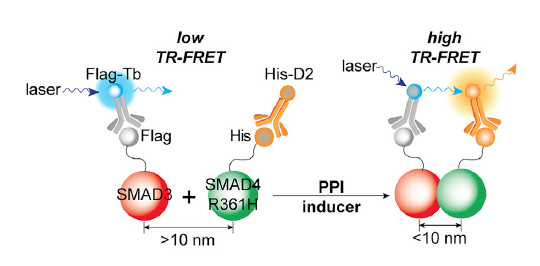
One of them, called Ro-31-8220, previously attracted some attention from cancer researchers based on its ability to inhibit another enzyme (protein kinase C), but the Emory team amassed evidence pointing to a direct effect on the SMAD3-SMAD4 interaction. They showed that Ro-31-8220 could restore inhibition by TGF-beta in colon cancer cells.
“This compound itself will serve as a structural starting point for us to develop the next generation of SMAD4-mutant PPI activators with enhanced specificity, potency, and desired pharmacological properties,” Fu says.
First author Tang is a visiting graduate student from the Emory University–Xi’an Jiaotong University Health Science Center exchange program. The research was supported by the National Cancer Institute (U01CA217875, P50CA217691, P30CA138292), the Georgia Clinical & Translational Science Alliance, the Georgia Research Alliance and an I3 award (Imagine, Innovate and Impact) from Emory University School of Medicine.

